Key Points
-
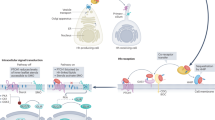
Secretion of cholesterol-modified and palmitate-modified Hedgehog (Hh) might occur through the formation of multimers or by association with lipoproteins. Secretion is an active process that depends on the 12-transmembrane-domain protein Dispatched, a member of the resistance-nodulation division (RND) family of proton-driven transporters.
-
Hh signals by binding to Patched, another RND family member, and by preventing Patched repression of the G-protein-coupled receptor Smoothened. Smoothened activity is likely to be regulated by the binding of one or more small lipophilic molecules: candidates include vitamin D3 and several oxysterols.
-
Patched might repress Smoothened by regulating the availability or synthesis of small lipophilic Smoothened inhibitors.
-
Intracellular cholesterol transport is tightly regulated and the biosynthesis of different cholesterol derivatives occurs at specific subcellular locations. The similarity of Patched to the cholesterol-mobilizing transporter NPC1 suggests that it might be involved in intracellular trafficking of cholesterol or its derivatives.
Abstract
The identification of endogenous sterol derivatives that modulate the Hedgehog (Hh) signalling pathway has begun to suggest testable hypotheses for the cellular biological functions of Patched, and for the lipoprotein association of Hh. Progress in the field of intracellular sterol trafficking has emphasized how tightly the distribution of intracellular sterol is controlled, and suggests that the synthesis of sterol derivatives can be influenced by specific sterol-delivery pathways. The combination of this field with Hh studies will rapidly give us a more sophisticated understanding of both the Hh signal-transduction pathway and the cell biology of sterol metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
van den Brink, G. R. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol. Rev. 87, 1343–1375 (2007).
Palma, V. et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132, 335–344 (2005).
Crompton, T., Outram, S. V. & Hager-Theodorides, A. L. Sonic hedgehog signalling in T-cell development and activation. Nature Rev. Immunol. 7, 726–735 (2007).
Cooper, M. K. et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nature Genet. 33, 508–513 (2003).
Ming, J. E., Roessler, E. & Muenke, M. Human developmental disorders and the Sonic hedgehog pathway. Mol. Med. Today 4, 343–349 (1998).
Berman, D. M. et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425, 846–851 (2003).
Datta, S. & Datta, M. W. Sonic Hedgehog signaling in advanced prostate cancer. Cell. Mol. Life Sci. 63, 435–448 (2006).
Wetmore, C. Sonic hedgehog in normal and neoplastic proliferation: insight gained from human tumors and animal models. Curr. Opin. Genet. Dev. 13, 34–42 (2003).
Ruizi Altaba, A., Sanchez, P. & Dahmane, N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nature Rev. Cancer 2, 361–372 (2002).
Porter, J. A., Young, K. E. & Beachy, P. A. Cholesterol modification of Hedgehog signaling proteins in animal development. Science 274, 255–259 (1996).
Porter, J. A. et al. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell 86, 21–34 (1996).
Pepinsky, R. B. et al. Identification of a palmitic acid-modified form of human Sonic hedgehog. J. Biol. Chem. 273, 14037–14045 (1998).
Chamoun, Z. et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science 293, 2080–2084 (2001).
Amanai, K. & Jiang, J. Distinct roles of Central missing and Dispatched in sending the Hedgehog signal. Development 128, 5119–5127 (2001).
Lee, J. D. & Treisman, J. E. Sightless has homology to transmembrane acyltransferases and is required to generate active Hedgehog protein. Curr. Biol. 11, 1147–1152 (2001).
Micchelli, C. A., The, I., Selva, E., Mogila, V. & Perrimon, N. Rasp, a putative transmembrane acyltransferase, is required for Hedgehog signaling. Development 129, 843–851 (2002).
Gallet, A., Rodriguez, R., Ruel, L. & Therond, P. P. Cholesterol modification of Hedgehog is required for trafficking and movement, revealing an asymmetric cellular response to Hedgehog. Dev. Cell 4, 191–204 (2003).
Gallet, A., Ruel, L., Staccini-Lavenant, L. & Therond, P. P. Cholesterol modification is necessary for controlled planar long-range activity of Hedgehog in Drosophila epithelia. Development 133, 407–418 (2006). Shows that cholesterol modification of Hh is required for its long-range signalling activity and for its incorporation into high molecular weight multimers in vivo.
Chen, M. H., Li, Y. J., Kawakami, T., Xu, S. M. & Chuang, P. T. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 18, 641–659 (2004). Shows that lipid modification of Sonic Hh is necessary for incorporation into high molecular weight multimeric complexes and that incorporation into these complexes promotes Hh signalling activity.
Lee, J. D. et al. An acylatable residue of Hedgehog is differentially required in Drosophila and mouse limb development. Dev. Biol. 233, 122–136 (2001).
Rietveld, A., Neutz, S., Simons, K. & Eaton, S. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J. Biol. Chem. 274, 12049–12054 (1999).
Taipale, J. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009 (2000).
Feng, J. et al. Synergistic and antagonistic roles of the Sonic hedgehog N- and C-terminal lipids. Development 131, 4357–4370 (2004).
Burke, R. et al. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99, 803–815 (1999).
Ma, Y. et al. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell 111, 63–75 (2002).
Piddock, L. J. Multidrug-resistance efflux pumps — not just for resistance. Nature Rev. Microbiol. 4, 629–636 (2006).
Chang, T. Y., Chang, C. C., Ohgami, N. & Yamauchi, Y. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22, 129–157 (2006).
Zeng, X. et al. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411, 716–720 (2001).
Callejo, A., Torroja, C., Quijada, L. & Guerrero, I. Hedgehog lipid modifications are required for Hedgehog stabilization in the extracellular matrix. Development 133, 471–483 (2006).
Panáková, D., Sprong, H., Marois, E., Thiele, C. & Eaton, S. Lipoprotein particles carry lipid-linked proteins and are required for long-range Hedgehog and Wingless signalling. Nature 435, 58–65 (2005). Shows that D. melanogaster Wingless, Hh and several GPI-linked proteins bind to lipoprotein particles. RNA-mediated knockdown of Lipophorin reduces the range of both Wingless and Hh signalling.
van der Horst, D. J., van Hoof, D., van Marrewijk, W. J. & Rodenburg, K. W. Alternative lipid mobilization: the insect shuttle system. Mol. Cell Biochem. 239, 113–119 (2002).
Arrese, E. L. et al. Lipid storage and mobilization in insects: current status and future directions. Insect Biochem. Mol. Biol. 31, 7–17 (2001).
Kutty, R. K. et al. Molecular characterization and developmental expression of a retinoid- and fatty acid-binding glycoprotein from Drosophila. A putative lipophorin. J. Biol. Chem. 271, 20641–20649 (1996).
Babin, P. J., Bogerd, J., Kooiman, F. P., Van Marrewijk, W. J. & Van der Horst, D. J. Apolipophorin II/I, apolipoprotein B, vitellogenin, and microsomal triglyceride transfer protein genes are derived from a common ancestor. J. Mol. Evol. 49, 150–160 (1999).
Eugster, C., Panakova, D., Mahmoud, A. & Eaton, S. Lipoprotein–heparan sulfate interactions in the Hedgehog pathway. Dev. Cell 13, 57–71 (2007).
Li, Y., Zhang, H., Litingtung, Y. & Chiang, C. Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc. Natl Acad. Sci. USA 103, 6548–6553 (2006).
The, I., Bellaiche, Y. & Perrimon, N. Hedgehog movement is regulated through tout velu-dependent synthesis of a heparan sulfate proteoglycan. Mol. Cell 4, 633–639 (1999).
Takei, Y., Ozawa, Y., Sato, M., Watanabe, A. & Tabata, T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 131, 73–82 (2004).
Han, C., Belenkaya, T. Y., Wang, B. & Lin, X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development 131, 601–611 (2004).
Taipale, J., Cooper, M. K., Maiti, T. & Beachy, P. A. Patched acts catalytically to suppress the activity of Smoothened. Nature 418, 892–897 (2002).
Chen, J. K., Taipale, J., Cooper, M. K. & Beachy, P. A. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 16, 2743–2748 (2002).
Cooper, M. K., Porter, J. A., Young, K. E. & Beachy, P. A. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science 280, 1603–1607 (1998).
Incardona, J. P., Gaffield, W., Kapur, R. P. & Roelink, H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development 125, 3553–3562 (1998).
Frank-Kamenetsky, M. et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J. Biol. 1, 10 (2002).
Chen, J. K., Taipale, J., Young, K. E., Maiti, T. & Beachy, P. A. Small molecule modulation of Smoothened activity. Proc. Natl Acad. Sci. USA 99, 14071–14076 (2002).
Strutt, H. et al. Mutations in the sterol-sensing domain of Patched suggest a role for vesicular trafficking in Smoothened regulation. Curr. Biol. 11, 608–613 (2001).
Paulsen, I. T., Brown, M. H. & Skurray, R. A. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60, 575–608 (1996).
Kelley, R. I. & Herman, G. E. Inborn errors of sterol biosynthesis. Annu. Rev. Genomics Hum. Genet. 2, 299–341 (2001).
Porter, F. D. Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr. Opin. Pediatr. 15, 607–613 (2003).
Tadjuidje, E. & Hollemann, T. Cholesterol homeostasis in development: the role of Xenopus 7-dehydrocholesterol reductase (Xdhcr7) in neural development. Dev. Dyn. 235, 2095–2110 (2006).
Engelking, L. J. et al. Severe facial clefting in Insig-deficient mouse embryos caused by sterol accumulation and reversed by lovastatin. J. Clin. Invest. 116, 2356–2365 (2006).
Wassif, C. A. et al. Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith–Lemli–Opitz syndrome. Hum. Mol. Genet. 10, 555–564 (2001).
Bijlsma, M. F. et al. Repression of smoothened by patched-dependent (pro-) vitamin D3 secretion. PLoS Biol. 4, e232 (2006). Shows that adding pro-vitamin D 3 inhibits Hh signalling in tissue-culture cells and in zebrafish embryos.
DeLuca, H. F. & Schnoes, H. K. Metabolism and mechanism of action of vitamin D. Annu. Rev. Biochem. 45, 631–666 (1976).
Haddad, J. G., Matsuoka, L. Y., Hollis, B. W., Hu, Y. Z. & Wortsman, J. Human plasma transport of vitamin D after its endogenous synthesis. J. Clin. Invest. 91, 2552–2555 (1993).
Dusso, A. S., Brown, A. J. & Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal Physiol. 289, F8–F28 (2005).
Zugmaier, G. et al. Growth-inhibitory effects of vitamin D analogues and retinoids on human pancreatic cancer cells. Br. J. Cancer 73, 1341–1346 (1996).
Colston, K. W., James, S. Y., Ofori-Kuragu, E. A., Binderup, L. & Grant, A. G. Vitamin D receptors and anti-proliferative effects of vitamin D derivatives in human pancreatic carcinoma cells in vivo and in vitro. Br. J. Cancer 76, 1017–1020 (1997).
Narvaez, C. J., Zinser, G. & Welsh, J. Functions of 1α,25-dihydroxyvitamin D3 in mammary gland: from normal development to breast cancer. Steroids 66, 301–308 (2001).
Beer, T. M. & Myrthue, A. Calcitriol in the treatment of prostate cancer. Anticancer Res. 26, 2647–2651 (2006).
Kha, H. T. et al. Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J. Bone Miner. Res. 19, 830–840 (2004).
Dwyer, J. R. et al. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol. Chem. 282, 8959–8968 (2007). Shows that oxysterols act through the Hh pathway at or above the level of Smoothened to exert their bone-promoting effects.
Corcoran, R. B. & Scott, M. P. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl Acad. Sci. USA 103, 8408–8413 (2006). Reports that both Hh signalling and proliferation in a medulloblastoma cell line require cholesterol biosynthesis.
Berman, D. M. et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 297, 1559–1561 (2002).
Frolov, A. et al. NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol-derived oxysterols. J. Biol. Chem. 278, 25517–25525 (2003).
Rohatgi, R., Milenkovic, L. & Scott, M. P. Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372–376 (2007). Shows that Patched localizes to primary cilia and prevents the accumulation of Smoothened. Addition of Sonic Hh removes Patched from the cilium and allows Smoothened accumulation.
Peet, D. J., Janowski, B. A. & Mangelsdorf, D. J. The LXRs: a new class of oxysterol receptors. Curr. Opin. Genet. Dev. 8, 571–575 (1998).
Fairn, G. D. & McMaster, C. R. Emerging roles of the oxysterol-binding protein family in metabolism, transport, and signaling. Cell. Mol. Life Sci. 65, 228–236 (2007).
Olkkonen, V. M. et al. The OSBP-related proteins (ORPs): global sterol sensors for co-ordination of cellular lipid metabolism, membrane trafficking and signalling processes? Biochem. Soc. Trans. 34, 389–391 (2006).
Radhakrishnan, A., Ikeda, Y., Kwon, H. J., Brown, M. S. & Goldstein, J. L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc. Natl Acad. Sci. USA 104, 6511–6518 (2007).
Soccio, R. E. & Breslow, J. L. Intracellular cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 24, 1150–1160 (2004).
Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nature Rev. Mol. Cell Biol. 9, 125–138 (2008).
Russell, D. W. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta 1529, 126–135 (2000).
Davies, J. P., Chen, F. W. & Ioannou, Y. A. Transmembrane molecular pump activity of Niemann–Pick C1 protein. Science 290, 2295–2298 (2000).
Strauss, J. F., 3rd, Kishida, T., Christenson, L. K., Fujimoto, T. & Hiroi, H. START domain proteins and the intracellular trafficking of cholesterol in steroidogenic cells. Mol. Cell Endocrinol. 202, 59–65 (2003).
Yang, H. Nonvesicular sterol transport: two protein families and a sterol sensor? Trends Cell Biol. 16, 427–432 (2006).
Garver, W. S. & Heidenreich, R. A. The Niemann–Pick C proteins and trafficking of cholesterol through the late endosomal/lysosomal system. Curr. Mol. Med. 2, 485–505 (2002).
Mukherjee, S. & Maxfield, F. R. Lipid and cholesterol trafficking in NPC. Biochim. Biophys. Acta 1685, 28–37 (2004).
Chang, T. Y. et al. Niemann–Pick type C disease and intracellular cholesterol trafficking. J. Biol. Chem. 280, 20917–20920 (2005).
Malerod, L., Juvet, L. K., Hanssen-Bauer, A., Eskild, W. & Berg, T. Oxysterol-activated LXRα/RXR induces hSR-BI-promoter activity in hepatoma cells and preadipocytes. Biochem. Biophys. Res. Commun. 299, 916–923 (2002).
Venkateswaran, A. et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRα. Proc. Natl Acad. Sci. USA 97, 12097–12102 (2000).
Huang, X., Suyama, K., Buchanan, J., Zhu, A. J. & Scott, M. P. A Drosophila model of the Niemann–Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development 132, 5115–5124 (2005).
Fluegel, M. L., Parker, T. J. & Pallanck, L. J. Mutations of a Drosophila NPC1 gene confer sterol and ecdysone metabolic defects. Genetics 172, 185–196 (2006).
Li, J., Brown, G., Ailion, M., Lee, S. & Thomas, J. H. NCR-1 and NCR-2, the C. elegans homologs of the human Niemann–Pick type C1 disease protein, function upstream of DAF-9 in the dauer formation pathways. Development 131, 5741–5752 (2004).
Griffin, L. D., Gong, W., Verot, L. & Mellon, S. H. Niemann–Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nature Med. 10, 704–711 (2004).
Xie, C., Richardson, J. A., Turley, S. D. & Dietschy, J. M. Cholesterol substrate pools and steroid hormone levels are normal in the face of mutational inactivation of NPC1 protein. J. Lipid Res. 47, 953–963 (2006).
Azhar, S., Leers-Sucheta, S. & Reaven, E. Cholesterol uptake in adrenal and gonadal tissues: the SR-BI and 'selective' pathway connection. Front. Biosci. 8, S998–S1029 (2003).
Kraemer, F. B. Adrenal cholesterol utilization. Mol. Cell Endocrinol. 265–266, 42–45 (2007).
Kraemer, F. B. et al. Hormone-sensitive lipase is required for high-density lipoprotein cholesteryl ester-supported adrenal steroidogenesis. Mol. Endocrinol. 18, 549–557 (2004).
Shen, W. J. et al. Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein: facilitation of cholesterol transfer in adrenal. J. Biol. Chem. 278, 43870–43876 (2003).
Li, H. et al. Hormone-sensitive lipase deficiency in mice causes lipid storage in the adrenal cortex and impaired corticosterone response to corticotropin stimulation. Endocrinology 143, 3333–3340 (2002).
Willnow, T. E. & Nykjaer, A. Pathways for kidney-specific uptake of the steroid hormone 25-hydroxyvitamin D3 . Curr. Opin. Lipidol. 13, 255–260 (2002).
Adams, J. S. et al. Novel regulators of vitamin D action and metabolism: Lessons learned at the Los Angeles zoo. J. Cell Biochem. 88, 308–314 (2003).
Adams, J. S. et al. Response element binding proteins and intracellular vitamin D binding proteins: novel regulators of vitamin D trafficking, action and metabolism. J. Steroid Biochem. Mol. Biol. 89–90(1–5), 461–465 (2004).
Wu, S. et al. Regulation of 1,25-dihydroxyvitamin D synthesis by intracellular vitamin D binding protein-1. Endocrinology 143, 4135 (2002).
Wu, S. et al. Intracellular vitamin D binding proteins: novel facilitators of vitamin D-directed transactivation. Mol. Endocrinol. 14, 1387–1397 (2000).
Incardona, J. P. et al. Cyclopamine inhibition of Sonic hedgehog signal transduction is not mediated through effects on cholesterol transport. Dev. Biol. 224, 440–452 (2000).
McCarthy, R. A., Barth, J. L., Chintalapudi, M. R., Knaak, C. & Argraves, W. S. Megalin functions as an endocytic sonic hedgehog receptor. J. Biol. Chem. 277, 25660–25667 (2002).
Morales, C. R. et al. Epithelial trafficking of Sonic hedgehog by megalin. J. Histochem. Cytochem. 54, 1115–1127 (2006).
Willnow, T. E. et al. Defective forebrain development in mice lacking gp330/megalin. Proc. Natl Acad. Sci. USA 93, 8460–8464 (1996).
Tam, S. P., Mok, L., Chimini, G., Vasa, M. & Deeley, R. G. ABCA1 mediates high-affinity uptake of 25-hydroxycholesterol by membrane vesicles and rapid efflux of oxysterol by intact cells. Am. J. Physiol. Cell Physiol. 291, C490–C502 (2006).
Denef, N., Neubuser, D., Perez, L. & Cohen, S. M. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell 102, 521–531 (2000).
Zhu, A. J., Zheng, L., Suyama, K. & Scott, M. P. Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 17, 1240–1252 (2003).
Huangfu, D. & Anderson, K. V. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl Acad. Sci. USA 102, 11325–11330 (2005).
Zhang, C., Williams, E. H., Guo, Y., Lum, L. & Beachy, P. A. Extensive phosphorylation of Smoothened in Hedgehog pathway activation. Proc. Natl Acad. Sci. USA 101, 17900–17907 (2004).
Apionishev, S., Katanayeva, N. M., Marks, S. A., Kalderon, D. & Tomlinson, A. Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction. Nature Cell Biol. 7, 86–92 (2005).
Zhao, Y., Tong, C. & Jiang, J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature 450, 252–258 (2007). In an elegant set of FRET experiments, the authors show that phosphorylation of a series of Ser residues in the Smoothened tail balances the positive charges on multiple adjacent Arg residues and changes tail conformation, leading to Smoothened dimerization and activation.
Lum, L. & Beachy, P. A. The Hedgehog response network: sensors, switches, and routers. Science 304, 1755–1759 (2004).
Kalderon, D. The mechanism of hedgehog signal transduction. Biochem. Soc. Trans. 33, 1509–1512 (2005).
Huangfu, D. & Anderson, K. V. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133, 3–14 (2006).
Acknowledgements
I am grateful to T. Kurzchalia and the members of my laboratory for critical comments on this work.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Interpro
OMIM
FURTHER INFORMATION
Glossary
- Inteins
-
(Protein introns). Enzymatically active domains that splice themselves out of the precursor protein, ligating the protein fragments (the 'exteins') on either side. Inteins can also ligate exteins in trans as well as in cis, and this has been exploited to modify proteins in vitro.
- Raft-lipid microdomains
-
Small phase-separated regions of the cell membranes that are rich in cholesterol and sphingolipids. Their affinity for specific transmembrane and lipid-linked proteins, and their ability to cluster to form higher order structures have been proposed to be important for the regulation of signalling and membrane trafficking.
- Cleft palate
-
A craniofacial abnormality that results from failure to fuse the left and right palatal shelves at the midline during embryogenesis. It can be caused by several environmental and genetic factors, including defects in Sonic Hh signalling.
- Dauer formation
-
When starved of nutrients, the nematode worm C. elegans progresses to a unique larval form called a dauer. Dauers are long-lived and resistant to environmental insults but do not reproduce. When conditions are favourable, dauer larvae re-enter reproductive development.
- ABC transporter
-
The many different proteins of the ABC transporter family use ATP to transport small molecules across the plasma membrane. ABCA1 has an important function in reverse cholesterol transport — effluxing cellular cholesterol to HDL particles. ABCG5 and G8 efflux cholesterol and plant sterols out of gut cells and into the gut lumen, regulating dietary sterol uptake.
Rights and permissions
About this article
Cite this article
Eaton, S. Multiple roles for lipids in the Hedgehog signalling pathway. Nat Rev Mol Cell Biol 9, 437–445 (2008). https://doi.org/10.1038/nrm2414
Issue Date:
DOI: https://doi.org/10.1038/nrm2414
This article is cited by
-
GPR161 structure uncovers the redundant role of sterol-regulated ciliary cAMP signaling in the Hedgehog pathway
Nature Structural & Molecular Biology (2024)
-
Hedgehog-Interacting Protein is a multimodal antagonist of Hedgehog signalling
Nature Communications (2021)
-
The regulation of IMF deposition in pectoralis major of fast- and slow- growing chickens at hatching
Journal of Animal Science and Biotechnology (2017)
-
Protein arginine methyltransferase 1 interacts with Gli1 and regulates its transcriptional activity
Tumor Biology (2016)
-
Smoothened regulation in response to Hedgehog stimulation
Frontiers in Biology (2015)