Key Points
-
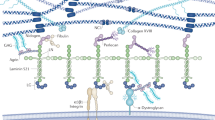
Basement membrane (BM) proteoglycans are more than charged filtering agents. These complex macromolecules can direct cell responses by a wide range of sophisticated mechanisms. BM proteoglycans are directly involved in regulating angiogenesis and, consequently, tumour progression, and their partial or total absence causes several congenital diseases that affect the cardiovascular, musculoskeletal and nervous systems.
-
Whereas the two heparan sulphate proteoglycans collagen XVIII and perlecan dictate BM stability and integrity in a variety of tissues, C-terminal-derived fragments of both collagen XVIII (endostatin) and perlecan (endorepellin) inhibit the growth of blood vessels by targeting tumour-associated endothelial cells. BM proteoglycans can have a bipolar activity — through the heparan sulphate chains they function as pro-angiogenic factors by modulating growth factor activity, whereas through their C-terminal angiostatic fragments they have the opposite function.
-
The mechanism of action of endostatin and endorepellin involves their specific interaction with two cell surface receptors, the α5β1 and the α2β1 integrins, respectively, through a process that ultimately disrupts the endothelial cell cytoskeleton and focal adhesions, thereby blocking cell migration and capillary morphogenesis. This seems to be the primary angiostatic function of these processed forms of BM proteoglycans.
-
The presence of these fragments in the urine and blood of normal and diseased patients indicate that they might exert a physiological role in vivo, and that, in the future, they could become biomolecular markers for certain diseases.
-
A number of future challenges include the precise delineation of their signalling networks and an improved understanding of their in vivo activities and interacting partners. By increasing our knowledge we will optimize the use of these endogenous angiogenesis inhibitors in the battle against cancer and other diseases where angiogenesis is dominant.
Abstract
The biology of basement membrane proteoglycans extends far beyond the original notion of anionic filters. These complex molecules have dual roles as structural constituents of basement membranes and functional regulators of several growth-factor signalling pathways. As such, they are involved in angiogenesis and, consequently, in tumour progression and their partial or total absence causes several congenital defects that affect the musculoskeletal, cardiovascular and nervous systems. New findings indicate a potential functional coupling between the intricate make-up of basement membrane proteoglycans and their ability to control important biological processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yurchenco, P. D., Amenta, P. S. & Patton, B. L. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 22, 521–538 (2004). An excellent and comprehensive review on basement membrane constituents.
Kanwar, Y. S. & Farquhar, M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc. Natl Acad. Sci. USA 76, 1303–1307 (1979).
Oh, S. P. et al. Isolation and sequencing of cDNAs for proteins with multiple domains of Gly-Xaa-Yaa repeats identify a distinct family of collagenous proteins. Proc. Natl Acad. Sci. USA 91, 4229–4233 (1994).
Rehn, M. & Pihlajaniemi, T. α1(XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution, and homology with type XV collagen. Proc. Natl Acad. Sci. USA 91, 4234–4238 (1994). References 3 and 4 provide the first reports on collagen XVIII.
Iozzo, R. V. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 67, 609–652 (1998).
Bezakova, G. & Ruegg, M. A. New insights into the roles of agrin. Nature Rev. Mol. Cell Biol. 4, 295–308 (2003).
Iozzo, R. V. & San Antonio, J. D. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J. Clin. Invest. 108, 349–355 (2001).
Li, D., Clark, C. C. & Myers, J. C. Basement membrane zone type XV collagen is a disulfide-bonded chondroitin sulfate proteoglycan in human tissues and cultured cells. J. Biol. Chem. 275, 22339–22347 (2000).
Marneros, A. G. & Olsen, B. R. The role of collagen-derived proteolytic fragments in angiogenesis. Matrix Biol. 20, 337–345 (2001).
Saarela, J., Rehn, M., Oikarinen, A., Autio-Harmainen, H. & Pihlajaniemi, T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am. J. Pathol. 153, 611–626 (1998).
Muragaki, Y. et al. Mouse Col18a1 is expressed in a tissue-specific manner as three alternative variants and is localized in basement membrane zones. Proc. Natl Acad. Sci. USA 92, 8763–8767 (1995).
Rehn, M. & Pihlajaniemi, T. Identification of three N-terminal ends of type XVIII collagen chains and tissue-specific differences in the expression of the corresponding transcripts. The longest form contains a novel motif homologous to rat and Drosophila frizzled proteins. J. Biol. Chem. 270, 4705–4711 (1995).
Halfter, W., Dong, S., Schurer, B. & Cole, G. J. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 273, 25404–25412 (1998). These authors discovered that collagen XVIII is a heparan sulphate proteoglycan.
Dong, G, Cole, G. J. & Halfter, W. Expression of collagen XVIII and localization of its glycosaminoglycan attachment sites. J. Biol. Chem. 278, 1700–1707 (2003).
Pufe, T. et al. Endostatin/collagen XVIII — an inhibitor of angiogenesis — is expressed in cartilage and fibrocartilage. Matrix Biol. 23, 267–276 (2004).
Amenta, P. S. et al. Proteoglycan-collagen XV in human tissues is seen linking banded collagen fibers subjacent to the basement membrane. J. Histochem. Cytochem. 53, 165–176 (2005).
Fukai, N. et al. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 21, 1535–1544 (2002).
Ylikärppä, R. et al. Lack of type XVIII collagen results in anterior ocular defects. FASEB J. 17, 2257–2259 (2003). By generating collagen-XVIII-null animals, these authors (references 17 and 18) provide compelling evidence for a principal role of collagen XVIII in eye development.
Marneros, A. G. et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 23, 89–99 (2004).
Sertie, A. L. et al. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome). Human Mol. Gen. 9, 2051–2058 (2000).
Suzuki, O. T. et al. Molecular analysis of collagen XVIII reveals novel mutations, presence of a third isoform, and possible genetic heterogeneity in Knobloch syndrome. Am. J. Hum. Genet. 71, 1320–1329 (2002).
Utriainen, A. et al. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Human Mol. Gen. 13, 2089–2099 (2004).
Moulton, K. S. et al. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation 110, 1330–1336 (2004).
Elamaa, H., Sormunen, R., Rehn, M., Soininen, R. & Pihlajaniemi, T. Endostatin overexpression specifically in the lens and skin leads to cataract and ultrastructural alterations in basement membranes. Am. J. Pathol. 166, 221–229 (2004).
Ackley, B. D. et al. The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J. Cell Biol. 152, 1219–1232 (2001).
Kliemann, S. E., Waetge, R. T., Suzuki, O. T., Passos-Bueno, M. R. & Rosemberg, S. Evidence of neuronal migration disorders in Knobloch syndrome: clinical and molecular analysis of two novel families. Am. J. Med. Genet. 119, 15–19 (2003).
Ackley, B. D. et al. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J. Neurosci. 23, 3577–3587 (2003).
Eklund, L. et al. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc. Natl Acad. Sci. USA 98, 1194–1199 (2001).
Ylikärppä, R. et al. Double knockout mice reveal a lack of major functional compensation between collagens XV and XVIII. Matrix Biol. 22, 443–448 (2003).
Noonan, D. M. et al. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J. Biol. Chem. 266, 22939–22947 (1991). The first complete characterization of the modular nature of perlecan protein core.
Iozzo, R. V. Perlecan: a gem of a proteoglycan. Matrix Biol. 14, 203–208 (1994).
Iozzo, R. V., Cohen, I. R., Grä ssel, S. & Murdoch, A. D. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem. J. 302, 625–639 (1994).
Dolan, M., Horchar, T., Rigatti, B. & Hassell, J. R. Identification of sites in domain I of perlecan that regulate heparan sulfate synthesis. J. Biol. Chem. 272, 4316–4322 (1997).
Friedrich, M. V. K. et al. Structural basis of glycosaminoglycan modification and of heterotypic interactions of perlecan domain V. J. Mol. Biol. 294, 259–270 (1999).
Rogalski, T. M., Williams, B. D., Mullen, G. P. & Moerman, D. G. Products of the unc-52 gene in Caenorhabditis elegans are homologous to the core protein of the mammalian basement membrane heparan sulfate proteoglycan. Genes Dev. 7, 1471–1484 (1993).
Cohen, I. R., Grä ssel, S., Murdoch, A. D. & Iozzo, R. V. Structural characterization of the complete human perlecan gene and its promoter. Proc. Natl Acad. Sci. USA 90, 10404–10408 (1993).
Friedrich, M. V. K., Schneider, M., Timpl, R. & Baumgartner, S. Perlecan domain V of Drosophila melanogaster – sequence, recombinant analysis and tissue expression. Eur. J. Biochem. 267, 3149–3159 (2000).
Iozzo, R. V. Biosynthesis of heparan sulfate proteoglycan by human colon carcinoma cells and its localization at the cell surface. J. Cell Biol. 99, 403–417 (1984).
Bix, G. & Iozzo, R. V. Matrix revolutions: 'tails' of basement-membrane components with angiostatic functions. Trends Cell Biol. 15, 52–60 (2005).
SundarRaj, N., Fite, D., Ledbetter, S., Chakravarti, S. & Hassell, J. R. Perlecan is a component of cartilage matrix and promotes chondrocyte attachment. J. Cell Sci. 108, 2663–2672 (1995).
Handler, M., Yurchenco, P. D. & Iozzo, R. V. Developmental expression of perlecan during murine embryogenesis. Dev. Dyn. 210, 130–145 (1997). Comprehensive analysis of perlecan expression in murine development.
French, M. M. et al. Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro. J. Cell Biol. 145, 1103–1115 (1999).
Melrose, J., Smith, S. & Whitelock, J. Perlecan immunolocalizes to perichondrial vessels and canals in human fetal cartilaginous primordia in early vascular and matrix remodeling events associated with diarthrodial joint development. J. Histochem. Cytochem. 52, 1405–1413 (2004).
Govindraj, P. et al. Isolation and identification of the major heparan sulfate proteoglycans in the developing bovine rib growth plate. J. Biol. Chem. 277, 19461–19469 (2002).
Iozzo, R. V. & Murdoch, A. D. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 10, 598–614 (1996).
Nugent, M. A., Nugent, H. M., Iozzo, R. V., Sanchack, K. & Edelman, E. R. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc. Natl Acad. Sci. USA 97, 6722–6727 (2000). Presents compelling evidence for a role of perlecan in vascular injury.
Tapanadechopone, P., Tumova, S., Jiang, X. & Couchman, J. R. Epidermal transformation leads to increased perlecan synthesis with heparin-binding-growth-factor affinity. Biochem. J. 355, 517–527 (2001).
Jiang, J. et al. Essential contribution of tumor-derived perlecan to epidermal tumor growth and angiogenesis. J. Histochem. Cytochem. 52, 1575–1590 (2004).
Hassell, J. R., Yamada, Y. & Arikawa-Hirasawa, E. Role of perlecan in skeletal development and diseases. Glycoconj. J. 19, 263–267 (2003).
Olsen, B. R. Life without perlecan has its problems. J. Cell Biol. 147, 909–911 (1999).
Costell, M. et al. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 147, 1109–1122 (1999).
Arikawa-Hirasawa, E., Watanabe, E., Takami, H., Hassell, J. R. & Yamada, Y. Perlecan is essential for cartilage and cephalic development. Nature Genet. 23, 354–358 (1999). References 51 and 52 report the first characterization of perlecan-deficient mice and describe multiple vascular and cartilage abnormalities.
Costell, M. et al. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ. Res. 91, 158–164 (2002).
González-Iriarte, M. et al. Development of the coronary arteries in a murine model of transposition of great arteries. J. Mol. Cell. Cardio. 35, 795–802 (2003).
Rossi, M. et al. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 22, 236–245 (2003).
Tran, P. -K. et al. Increased intimal hyperplasia and smooth muscle cell proliferation in transgenic mice with heparan sulfate-deficient perlecan. Circ. Res. 94, 550–558 (2004).
Zhou, Z. et al. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 64, 4699–4702 (2004). The phenotype of perlecan-null mice is indeed complex, as shown by additional characterization of perlecan-deficient animals (references 53 and 54), or transgenic animals in which the heparan sulphate chains of perlecan were specifically targeted (references 55–57).
Arikawa-Hirasawa, E. et al. Dyssegmental dysplasia, Silverman–Handmaker type, is caused by functional null mutations of the perlecan gene. Nature Genet. 27, 431–434 (2001).
Arikawa-Hirasawa, E., Wilcox, W. R. & Yamada, Y. Dyssegmental dysplasia, Silverman–Handmaker type: unexpected role of perlecan in cartilage development. Am. J. Med. Genet. 106, 254–257 (2001).
Nicole, S. et al. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz–Jampel syndrome (chondrodystrophic myotonia). Nature Genet. 26, 480–483 (2000).
Arikawa-Hirasawa, E. et al. Structural and functional mutations of the perlecan gene cause Schwartz–Jampel syndrome, with myotonic myopathy and chondrodysplasia. Am. J. Hum. Genet. 70, 1368–1375 (2002). References 58–61 show a direct involvement of perlecan gene mutations in causing two distinct human genetic syndromes.
Cartaud, A. et al. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. J. Cell Biol. 165, 505–515 (2004).
Arikawa-Hirasawa, E., Rossi, S. G., Rotundo, R. L. & Yamada, Y. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nature Neurosci. 5, 119–123 (2002).
Voigt, A., Pflanz, R., Schafer, U. & Jackle, H. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev. Dyn. 224, 403–412 (2002).
Park, Y. et al. Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev. Biol. 253, 247–257 (2003). Shows that perlecan is directly involved in modulating growth factors and morphogens during development.
Rogalski, T. M., Gilchrist, E. J., Mullen, G. P. & Moerman, D. G. Mutations in the unc-52 gene responsible for body wall muscle defects in adult Caenorhabditis elegans are located in alternatively spliced exons. Genetics 139, 159–169 (1995).
Mullen, G. P., Rogalski, T. M., Bush, J. A., Gorji, P. R. & Moerman, D. G. Complex patterns of alternative splicing mediate the spatial and temporal distribution of perlecan/UNC-52 in Caenorhabditis elegans. Mol. Biol. Cell 10, 3205–3221 (1999).
Merz, D. C., Alves, G., Kawano, T., Zheng, H. & Culotti, J. G. UNC-52/perlecan affects gonadal leader cell migrations in C. elegans hermaphrodites through alterations in growth factor signaling. Dev. Biol. 256, 173–186 (2003).
O'Reilly, M. S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997).
Hohenester, E., Sasaki, T., Olsen, B. R. & Timpl, R. Crystal structure of the angiogenesis inhibitor endostatin at 1.5 Å resolution. EMBO J. 17, 1656–1664 (1998). An important paper that reports the solution structure of the C-terminal end of collagen XVIII, endostatin.
Sasaki, T. et al. Structural basis and potential role of heparin/heparan sulfate binding to the angiogenesis inhibitor endostatin. EMBO J. 18, 6240–6248 (1999).
Kreuger, J. et al. Role of heparan sulfate domain organization in endostatin inhibition of endothelial cell function. EMBO J. 21, 6303–6311 (2002).
Boehm, T., Folkman, J., Browder, T. & O'Reilly, M. S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 390, 404–407 (1997).
Sasaki, T. et al. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 17, 4249–4256 (1998).
Yamaguchi, N. et al. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J. 18, 4414–4423 (1999).
Citrin, D. et al. In vivo tumor imaging in mice with near-infrared labeled endostatin. Mol. Can. Ther. 3, 481–488 (2004). Reports clear evidence that endostatin targets the tumour vasculature.
Ramchandran, R. et al. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem. Biophys. Res. Commun. 255, 735–739 (1999).
Sasaki, T. et al. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J. Mol. Biol. 301, 1179–1190 (2000).
Bilbe, G. et al. Restin: a novel intermediate filament-associated protein highly expressed in the Reed–Sternberg cells of Hodgkin's disease. EMBO J. 11, 2103–2113 (1992).
Gaetzner, S. et al. Endostatin's heparan sulfate-binding site is essential for inhibition of angiogenesis and enhances in situ binding to capillary-like structures in bone explants. Matrix Biol. 23, 557–561 (2005).
Mongiat, M., Sweeney, S., San Antonio, J. D., Fu, J. & Iozzo, R. V. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 278, 4238–4249 (2003). The first report of endorepellin as an angiostatic factor.
Bix, G. et al. Endoprepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin. J. Cell Biol. 166, 97–109 (2004). This paper addresses the mechanism of action of endorepellin.
Miosge, N., Simniok, T., Sprysch, P. & Herken, R. The collagen type XVIII endostatin domain is co-localized with perlecan in basement membranes in vivo. J. Histochem. Cytochem. 51, 285–296 (2003).
Gonzalez, E. M. et al. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J. Biol. Chem. 280, 7080–7087 (2005).
Hohenester, E., Tisi, D., Talts, J. F. & Timpl, R. The crystal structure of a laminin G-like module reveals the molecular basis of α-dystroglycan binding to laminins, perlecan, and agrin. Mol. Cell 4, 783–792 (1999).
Tisi, D., Talts, J. F., Timpl, R. & Hohenester, E. Structure of the C-terminal laminin G-like domain pair of the laminin α2 chain harbouring binding sites for α-dystroglycan and heparin. EMBO J. 19, 1432–1440 (2000).
Wizemann, H. et al. Distinct requirements for heparin and α-dystroglycan binding revealed by structure-based mutagenesis of the laminin α2 LG4–LG5 domain pair. J. Mol. Biol. 332, 635–642 (2003).
Rudenko, G., Hohenester, E. & Muller, Y. A. LG/LNS domains: multiple functions — one business end? Trends Biochem. Sci. 26, 363–368 (2001).
Oda, O. et al. Purification and characterization of perlecan fragment in urine of end-stage renal failure patients. Clin. Chim. Acta 255, 119–132 (1996).
Vuadens, F. et al. Identification of biologic markers of the premature rupture of fetal membranes: proteomic approach. Proteomics 3, 1521–1525 (2003).
Adkins, J. N. et al. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol. Cell. Proteom. 1, 947–955 (2002).
Karumanchi, S. A. et al. Cell surface glypicans are low-affinity endostatin receptors. Mol. Cell 7, 811–822 (2001).
Ortega, N. & Werb, Z. New functional roles for non-collagenous domains of basement membrane collagens. J. Cell Sci. 115, 4201–4214 (2002).
Sottile, J. Regulation of angiogenesis by extracellular matrix. Biochim. Biophys. Acta 1654, 13–22 (2004).
Rehn, M. et al. Interaction of endostatin with integrins implicated in angiogenesis. Proc. Natl Acad. Sci. USA 98, 1024–1029 (2001).
Wickström, S. A., Alitalo, K. & Keski-Oja, J. Endostatin associates with integrin α5β1 and caveolin-1, and activates Src via a tyrosyl phosphatase-dependent pathway in human endothelial cells. Cancer Res. 62, 5580–5589 (2002).
Wickström, S. A., Alitalo, K. & Keski-Oja, J. Endostatin associates with lipid rafts and induces reorganization of the actin cytoskeleton via down-regulation of RhoA activity. J. Biol. Chem. 278, 37895–37901 (2003). References 96 and 97 provide convincing evidence for the binding of endostatin to α 5 β 1 integrin and elucidate its mechanism of action.
Sudhakar, A. et al. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc. Natl Acad. Sci. USA 100, 4766–4771 (2003).
Guo, W. & Giancotti, F. G. Integrin signalling during tumour progression. Nature Rev. Mol. Cell Biol. 5, 816–826 (2004). Excellent review on integrin signalling and cancer.
Abdollahi, A. H. P. et al. Endostatin's antioangiogenic signaling network. Mol. Cell 13, 649–663 (2004).
Emsley, J., Knight, C. G., Farndale, R. W., Barnes, M. J. & Liddington, R. C. Structural basis of collagen recognition by integrin α2β1 . Cell 101, 47–56 (2000).
Whelan, M. C. & Senger, D. R. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J. Biol. Chem. 278, 327–334 (2003).
Sweeney, S. M. et al. Angiogenesis in collagen I requires α2β1 ligation of a GFP*GER sequence and possible p38 MAPK activation and focal adhesion disassembly. J. Biol. Chem. 278, 30516–30524 (2003). References 102 and 103 investigate the role of collagen in inducing vascular morphogenesis.
Keezer, S. M. et al. Angiogenesis inhibitors target the endothelial cell cytoskeleton through altered regulation of heat shock protein 27 and cofilin. Cancer Res. 63, 6405–6412 (2003).
Guex, N. & Peitsch, M. C. SWISS-MODEL and Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Aricescu, A. R., McKinnell, I. W., Halfter, W. & Stoker, A. W. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase σ. Mol. Cell Biol. 22, 1881–1892 (2002).
van Horssen, J. et al. Collagen XVIII: a novel heparan sulfate proteoglycan associated with vascular amyloid depositions and senile plaques in Alzheimer's disease brains. Brain Pathol. 12, 456–462 (2002).
Bengtsson, E. et al. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J. Biol. Chem. 277, 15061–15068 (2002).
Knox, S., Merry, C., Stringer, S., Melrose, J. & Whitelock, J. Not all perlecans are created equal. Interactions with fibroblast growth factor (FGF) 2 and FGF receptors. J. Biol. Chem. 277, 14657–14665 (2002).
Xu, Y., Liu, Y. J. & Yu, Q. Angiopoietin-3 is tethered on the cell surface via heparan sulfate proteoglycans. J. Biol. Chem. 279, 41179–41188 (2004).
Tiedemann, K. et al. Microfibrils at basement membrane zones interact with perlecan via fibrillin-1. J. Biol. Chem. 280, 11404–11412 (2005).
Hummel, S. et al. Extracellular matrices of the avian ovarian follicle. Molecular characterization of chicken perlecan. J. Biol. Chem. 279, 23486–23494 (2004).
Mongiat, M. et al. The protein core of the proteoglycan perlecan binds specifically to fibroblast growth factor-7. J. Biol. Chem. 275, 7095–7100 (2000).
Göhring, W., Sasaki, T., Heldin, C. H. & Timpl, R. Mapping of the binding of platelet-derived growth factor to distinct domains of the basement membrane proteins BM-40 and perlecan and distinction from the BM-40 collagen-binding epitope. Eur. J. Biochem. 255, 60–66 (1998).
Hopf, M., Göhring, W., Kohfeldt, E., Yamada, Y. & Timpl, R. Recombinant domain IV of perlecan binds to nidogens, laminin–nidogen complex, fibronectin, fibulin-2 and heparin. Eur. J. Biochem. 259, 917–925 (1999).
Brown, J. C., Sasaki, T., Göhring, W., Yamada, E. & Timpl, R. The C-terminal domain V of perlecan promotes β1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur. J. Biochem. 250, 39–46 (1997).
Talts, J. F., Andac, Z., Göhring, W., Brancaccio, A. & Timpl, R. Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, α-dystroglycan and several extracellular matrix proteins. EMBO J. 18, 863–870 (1999).
Mongiat, M. et al. Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis. J. Biol. Chem. 278, 17491–17499 (2003).
Gonzalez, E. M., Mongiat, M., Slater, S. J., Baffa, R. & Iozzo, R. V. A novel interaction between perlecan protein core and progranulin: Potential effects on tumor growth. J. Biol. Chem. 278, 38113–38116 (2003).
Acknowledgements
I thank C. C. Clark and J. Hassell for critical reading of this manuscript, G. Bix and C. C. Reed for help with the illustrations and valuable comments, K. Camphausen for generously providing Fig. 3 and ART (Advanced Research Technologies) Inc. for the help with computer imaging. I apologize for failing to cite all relevant studies in the field because of space constraints. The original research was supported in part by the National Institutes of Health, and by the Commonwealth of Pennsylvania.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Related links
Related links
DATABASES
OMIM
Swiss-Prot
Flybase
FURTHER INFORMATION
Glossary
- BASEMENT MEMBRANE
-
A thin, complex extracellular matrix that separates endothelial and epithelial cells from their subjacent connective tissues. It is composed of various collagens, proteoglycans and adhesive glycoproteins.
- HEPARAN SULPHATE PROTEOGLYCAN
-
A specialized protein that has the glycosaminoglycan heparan sulphate covalently attached to a serine residue within a region often enriched in acidic amino acid residues.
- MULTIPLEXINS
-
(Multiple triple helix domains and interruptions.) This collagen subfamily comprises types XV and XVIII, which contain several interruptions in the central triple-helical (Gly–X–Y motif) domain and a unique Cterminal (NC1) domain. The interruptions generate flexible regions that enable the formation of polymers different to those generated by the fibrillar collagen types I, II, III, V and XI.
- NEUROMUSCULAR JUNCTION
-
The site of contact between the terminal of a motor neuron and the membrane of a muscle fibre. Nerve impulses are transmitted across the gap by diffusion of a transmitter.
- BASEMENT MEMBRANE ZONE
-
A region contiguous with the basement membrane where various proteins function as the anchoring structures to the subjacent connective tissue.
- FIBROCARTILAGE
-
A specialized form of cartilage composed of bundles of thick, clearly-defined collagen fibres, which is found in symphyseal joints (such as vertebrae) and in intervertebral and articular discs. It withstands tension and pressure, and absorbs shocks.
- CHOROID PLEXI
-
Collections of villous-like processes at select sites in the ventricular system of the brain. These processes contain a special secretory epithelium that secretes cerebrospinal fluid.
- INTEGRINS
-
A large family of heterodimeric transmembrane proteins that function as receptors for cell-adhesion molecules.
- GROWTH PLATE
-
An area of developing cartilaginous tissue near the ends of long bones, between the widened part of the shaft (the metaphysis) and the end (epiphysis) of the bone. The growth plate regulates and helps determine the length and shape of the mature bone.
- WNT PROTEINS
-
A family of highly conserved, secreted signalling molecules that regulate cell–cell interactions during embryogenesis.
- MATRIX METALLOPROTEINASES (MMPs).
-
A large family of secreted or transmembrane metal-dependent enzymes that collectively can digest all extracellular matrix and basement membrane constituents. They are active during tissue remodelling and angiogenesis.
- XENOGRAFT
-
Tissue or organ graft between species. These grafts are usually rejected unless immunocompromised animals are used.
- LG
-
(Laminin-like globular domain). LG modules are present in a number of basement membrane constituent proteins including laminin, perlecan and agrin. They fold into individual units and often possess important biological activity, as in the case of perlecan LG3.
- STRESS FIBRES
-
Bundles of parallel filaments that contain F-actin and other contractile molecules. They often stretch between cell attachments as if under stress.
- COLLAGEN SANDWICH
-
A widely used in vitro angiogenic assay in which endothelial cells are cultured between two layers of collagen type I. Within hours, visible capillary-like structures are formed.
- MATRIGEL
-
The extracellular matrix secreted by the Engelbrecht–Holm–Swarm mouse sarcoma cell line. It contains laminin, collagen IV, nidogen/entactin and proteoglycans, and therefore resembles the basement membrane.
- LIPID RAFTS
-
Rich in cholesterol, glycosphingolipids, glycosylphosphatidylinositol-anchored proteins and signalling molecules, these membrane microdomains function as signalling platforms.
- FOCAL ADHESION
-
An integrin-mediated cell–substrate adhesion structure that anchors the ends of actin filaments (stress fibres) and mediates strong attachments to substrates. It also functions as an integrin signalling platform.
- I DOMAIN
-
A ∼200 amino-acid inserted domain in the N-terminal region of the α2 and α1 integrin subunits that specifically binds various collagens. The binding is concentration-dependent and requires manganese and magnesium ions. The I domain is also known as MIDAS, for metal ion-dependent adhesion site.
Rights and permissions
About this article
Cite this article
Iozzo, R. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol 6, 646–656 (2005). https://doi.org/10.1038/nrm1702
Issue Date:
DOI: https://doi.org/10.1038/nrm1702
This article is cited by
-
Tremella fuciformis polysaccharides alleviates UV-provoked skin cell damage via regulation of thioredoxin interacting protein and thioredoxin reductase 2
Photochemical & Photobiological Sciences (2023)
-
Characterization and antioxidant activities of glycosaminoglycans from dried leech
Glycoconjugate Journal (2023)
-
Defective perlecan-associated basement membrane regeneration and altered modulation of transforming growth factor beta in corneal fibrosis
Cellular and Molecular Life Sciences (2022)
-
Structural analysis of glycosaminoglycans from Oviductus ranae
Glycoconjugate Journal (2021)
-
The CCN2/CTGF interactome: an approach to understanding the versatility of CCN2/CTGF molecular activities
Journal of Cell Communication and Signaling (2021)