Key Points
-
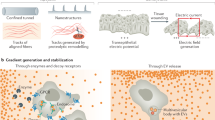
Motile eukaryotic cells such as Dictyostelium discoideum and human neutrophils extend pseudopodia with a typical 1-minute life cycle. In a uniform gradient of chemoattractant, these pseudopodia are formed in random directions.
-
During chemotaxis in a gradient of chemoattractant, the spatial and temporal aspects of the chemoattractant concentration are processed, leading to pseudopod extension at the leading edge, retraction of the uropod at the back of the cell, and suppression of lateral pseudopodia.
-
The chemoattractant binds to seven-transmembrane-spanning serpentine receptors, and activates heterotrimeric G-proteins and small GTP-binding proteins of the Rho/Rac class, which leads to the activation of phosphatidylinositol 3-kinase (PI3K) and guanylyl cyclase.
-
At the leading edge Rho/Rac proteins are activated. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) accumulates here as a result of the activity of PI3K that translocates from the cytosol, whereas the PtdIns(3,4,5)P3-degrading enzyme PTEN (phosphatase and tensin homologue) dissociates from the membrane at the leading edge. Rho/Rac proteins and PtdIns(3,4,5)P3-binding proteins induce actin polymerization and pseudopod formation.
-
At the sides and the back of the cell, myosin filaments are formed, which generate the power to retract the uropod, and also inhibit the formation of pseudopodia at the sides of the cell. In D. discoideum this is mediated predominantly by cyclic GMP, whereas in neutrophils, a Rho kinase induces myosin filaments.
Abstract
During random locomotion, human neutrophils and Dictyostelium discoideum amoebae repeatedly extend and retract cytoplasmic processes. During directed cell migration — chemotaxis — these pseudopodia form predominantly at the leading edge in response to the local accumulation of certain signalling molecules. Concurrent changes in actin and myosin enable the cell to move towards the stimulus. Recent studies are beginning to identify an intricate network of signalling molecules that mediate these processes, and how these molecules become localized in the cell is now becoming clear.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baggiolini, M. Chemokines and leukocyte traffic. Nature 392, 565–568 (1998).
Campbell, J. J. & Butcher, E. C. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12, 336–341 (2000).
Crone, S. A. & Lee, K. F. The bound leading the bound: target-derived receptors act as guidance cues. Neuron 36, 333–335 (2002).
Iijima, M., Huang, Y. E. & Devreotes, P. Temporal and spatial regulation of chemotaxis. Dev. Cell 3, 469–478 (2002).
Berg, H. C. A physicist looks at bacterial chemotaxis. Cold Spring Harb. Symp. Quant. Biol. 53, 1–9 (1988).
Bourret, R. B. & Stock, A. M. Molecular information processing: lessons from bacterial chemotaxis. J. Biol. Chem. 277, 9625–9628 (2002).
Zigmond, S. H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J. Cell Biol. 75, 606–616 (1977).
Devreotes, P. N. & Zigmond, S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu. Rev. Cell Biol. 4, 649–686 (1988).
Weiner, O. D. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr. Opin. Cell Biol. 14, 196–202 (2002).
Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc. Natl Acad. Sci. USA 101, 8951–8956 (2004).
Mato, J. M., Losada, A., Nanjundiah, V. & Konijn, T. M. Signal input for a chemotactic response in the cellular slime mold Dictyostelium discoideum. Proc. Natl Acad. Sci. USA 72, 4991–4993 (1975).
Stock, J. Sensitivity, cooperativity and gain in chemotaxis signal transduction. Trends Microbiol. 7, 1–4 (1999).
Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Postma, M., Bosgraaf, L., Loovers, H. M. & Van Haastert, P. J. M. Chemotaxis: signalling modules join hands at front and tail. EMBO Rep. 5, 35–40 (2004). The authors present a concept for chemotaxis based on the diffusion properties of signalling molecules, and their organization in several modules that regulate actin and myosin.
Pollard, T. D. & Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003). An excellent review on the regulation of actin filaments in motile cells by the Arp2/3 complex, profilin, capping proteins, WASP and WAVE/SCAR.
Swanson, J. A. & Taylor, D. L. Local and spatially coordinated movements in Dictyostelium discoideum amoebae during chemotaxis. Cell 28, 225–232 (1982).
Varnum-Finney, B., Edwards, K. B., Voss, E. & Soll, D. R. Amebae of Dictyostelium discoideum respond to an increasing temporal gradient of the chemoattractant cAMP with a reduced frequency of turning: evidence for a temporal mechanism in ameboid chemotaxis. Cell Motil. Cytoskeleton 8, 7–17 (1987).
Baldauf, S. L., Roger, A. J., Wenk-Siefert, I. & Doolittle, W. F. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 290, 972–977 (2000).
Wessels, D., Vawter-Hugart, H., Murray, J. & Soll, D. R. Three-dimensional dynamics of pseudopod formation and the regulation of turning during the motility cycle of Dictyostelium. Cell Motil. Cytoskeleton 27, 1–12 (1994).
Postma, M. et al. Uniform cAMP stimulation of Dictyostelium cells induces localized patches of signal transduction and pseudopodia. Mol. Biol. Cell 14, 5019–5027 (2003).
Wu, L., Valkema, R., Van Haastert, P. J. & Devreotes, P. N. The G protein β subunit is essential for multiple responses to chemoattractants in Dictyostelium. J. Cell Biol. 129, 1667–1675 (1995).
Insall, R. H., Soede, R. D., Schaap, P. & Devreotes, P. N. Two cAMP receptors activate common signaling pathways in Dictyostelium. Mol. Biol. Cell 5, 703–711 (1994).
Kriebel, P. W., Barr, V. A. & Parent, C. A. Adenylyl cyclase localization regulates streaming during chemotaxis. Cell 112, 549–560 (2003).
Soll, D. R., Wessels, D., Heid, P. J. & Zhang, H. A contextual framework for characterizing motility and chemotaxis mutants in Dictyostelium discoideum. J. Muscle Res. Cell Motil. 23, 659–672 (2002).
Soll, D. R. The use of computers in understanding how animal cells crawl. Int. Rev. Cytol. 163, 43–104 (1995).
Postma, M. et al. Sensitization of Dictyostelium chemotaxis by PI3-kinase mediated self-organizing signalling patches. J. Cell Sci. 117, 2925–2935 (2004).
Varnum, B. & Soll, D. R. Effects of cAMP on single cell motility in Dictyostelium. J. Cell Biol. 99, 1151–1155 (1984).
Verkhovsky, A. B., Svitkina, T. M. & Borisy, G. G. Self-polarization and directional motility of cytoplasm. Curr. Biol. 9, 11–20 (1999).
Bretschneider, T. et al. Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr. Biol. 14, 1–10 (2004).
Misteli, T. The concept of self-organization in cellular architecture. J. Cell Biol. 155, 181–185 (2001).
Nicolis, G. & Prigogine, I. Self-organization in nonequilibrium systems: from dissipative structures to order through fluctuations. (John Wiley & Sons, New York, 1977).
Bourne, H. R. & Weiner, O. A chemical compass. Nature 419, 21 (2002).
Devreotes, P. & Janetopoulos, C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445–20448 (2003). The authors define directional sensing as the ability of a cell to detect an asymmetric extracellular cue and generate an internal amplified response, whereas polarization is defined as the propensity of the cell to assume an asymmetric shape with a defined anterior and posterior. With these definitions many models for chemotaxis are evaluated.
Chen, L. et al. Two phases of actin polymerization display different dependencies on PI(3,4,5)P3 accumulation and have unique roles during chemotaxis. Mol. Biol. Cell 14, 5028–5037 (2003).
Van Duijn, B. & Van Haastert, P. J. Independent control of locomotion and orientation during Dictyostelium discoideum chemotaxis. J. Cell Sci. 102, 763–768 (1992).
Parent, C. A., Blacklock, B. J., Froehlich, W. M., Murphy, D. B. & Devreotes, P. N. G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81–91 (1998).
Klein, P. S. et al. A chemoattractant receptor controls development in Dictyostelium discoideum. Science 241, 1467–1472 (1988).
Saxe, C. L. 3rd, Johnson, R., Devreotes, P. N. & Kimmel, A. R. Multiple genes for cell surface cAMP receptors in Dictyostelium discoideum. Dev. Genet. 12, 6–13 (1991).
Youn, B. S., Mantel, C. & Broxmeyer, H. E. Chemokines, chemokine receptors and hematopoiesis. Immunol. Rev. 177, 150–174 (2000).
Le, Y., Murphy, P. M. & Wang, J. M. Formyl-peptide receptors revisited. Trends Immunol. 23, 541–548 (2002).
Maghazachi, A. A. G protein-coupled receptors in natural killer cells. J. Leukoc. Biol. 74, 16–24 (2003).
Kim, J. Y., Borleis, J. A. & Devreotes, P. N. Switching of chemoattractant receptors programs development and morphogenesis in Dictyostelium: receptor subtypes activate common responses at different agonist concentrations. Dev. Biol. 197, 117–128 (1998).
Dormann, D., Kim, J. Y., Devreotes, P. N. & Weijer, C. J. cAMP receptor affinity controls wave dynamics, geometry and morphogenesis in Dictyostelium. J. Cell Sci. 114, 2513–2523 (2001).
Kim, J. Y. et al. Phosphorylation of chemoattractant receptors is not essential for chemotaxis or termination of G-protein-mediated responses. J. Biol. Chem. 272, 27313–27318 (1997).
Richardson, R. M., Marjoram, R. J., Barak, L. S. & Snyderman, R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J. Immunol. 170, 2904–2911 (2003).
Zhang, N., Long, Y. & Devreotes, P. N. Gγ in Dictyostelium: its role in localization of Gβγ to the membrane is required for chemotaxis in shallow gradients. Mol. Biol. Cell 12, 3204–3213 (2001).
Janetopoulos, C., Jin, T. & Devreotes, P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science 291, 2408–2411 (2001).
Xu, J. et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114, 201–214 (2003). Expression of constitutively active and dominant-negative versions of various G proteins and GTPases in neutrophils indicate that chemoattractant-mediated signals segregate into two mutually exclusive pathways: G i –PtdIns(3,4,5)P 3 –Rac-dependent formation of F-actin at the front of cells and G 12/13 –RhoA-dependent formation of myosin-II filaments at the back.
Araki, T. et al. Developmentally and spatially regulated activation of a Dictyostelium STAT protein by a serpentine receptor. Embo J. 17, 4018–4028 (1998).
Milne, J. L., Wu, L., Caterina, M. J. & Devreotes, P. N. Seven helix cAMP receptors stimulate Ca2+ entry in the absence of functional G proteins in Dictyostelium. J. Biol. Chem. 270, 5926–5931 (1995).
Maeda, M. & Firtel, R. A. Activation of the mitogen-activated protein kinase ERK2 by the chemoattractant folic acid in Dictyostelium. J. Biol. Chem. 272, 23690–23695 (1997).
Meili, R. et al. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. Embo J. 18, 2092–2105 (1999).
Servant, G. et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040 (2000).
Zhou, K., Takegawa, K., Emr, S. D. & Firtel, R. A. A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vps34p PI 3-kinase homologs during growth and development. Mol. Cell. Biol. 15, 5645–5656 (1995).
Hirsch, E. et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science 287, 1049–1053 (2000).
Huang, Y. E. et al. Receptor mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol. Biol. Cell 14, 1913–1922 (2003).
Iijima, M. & Devreotes, P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109, 599–610 (2002). This paper and reference 60 characterize in detail the function and cellular localization of PI3K and Pten in D. discoideum cells. During chemotaxis PI3K is enriched at the leading edge and Pten accumulates at the posterior membrane of the cell.
Kalesnikoff, J. et al. The role of SHIP in cytokine-induced signaling. Rev. Physiol. Biochem. Pharmacol. 149, 87–103 (2003).
Loovers, H. et al. A diverse family of inositol 5-phosphatases playing a role in growth and development in Dictyostelium discoideum. J. Biol. Chem. 278, 5652–5658 (2002).
Funamoto, S., Meili, R., Lee, S., Parry, L. & Firtel, R. A. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109, 611–623 (2002).
Iijima, M., Huang, Y. E., Luo, H. R., Vazquez, F. & Devreotes, P. N. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J. Biol. Chem. 279, 16606–16613 (2004).
Wang, F. et al. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nature Cell Biol. 4, 513–518 (2002).
Millard, T. H., Sharp, S. J. & Machesky, L. M. Signalling to actin assembly via the WASP (Wiskott–Aldrich syndrome protein)-family proteins and the Arp2/3 complex. Biochem. J. 380, 1–17 (2004).
Machesky, L. M. & Insall, R. H. Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347–1356 (1998).
Blagg, S. L., Stewart, M., Sambles, C. & Insall, R. H. PIR121 regulates pseudopod dynamics and SCAR activity in Dictyostelium. Curr. Biol. 13, 1480–1487 (2003).
Bear, J. E., Rawls, J. F. & Saxe, C. L. 3rd. SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J. Cell Biol. 142, 1325–1335 (1998).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Gardiner, E. M. et al. Spatial and temporal analysis of Rac activation during live neutrophil chemotaxis. Curr. Biol. 12, 2029–2034 (2002).
Itoh, R. E. et al. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell Biol. 22, 6582–6591 (2002).
Wedlich-Soldner, R., Altschuler, S., Wu, L. & Li, R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299, 1231–1235 (2003).
Chubb, J. R. & Insall, R. H. Dictyostelium: an ideal organism for genetic dissection of Ras signalling networks. Biochim. Biophys. Acta 1525, 262–271 (2001).
Lim, C. J., Spiegelman, G. B. & Weeks, G. Cytoskeletal regulation by Dictyostelium Ras subfamily proteins. J. Muscle Res. Cell Motil. 23, 729–736 (2002).
Li, Z. et al. Directional sensing requires Gβγ-mediated PAK1 and PIXα-dependent activation of Cdc42. Cell 114, 215–227 (2003).
Welch, H. C., Coadwell, W. J., Stephens, L. R. & Hawkins, P. T. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 546, 93–97 (2003).
Welch, H. C. et al. P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell 108, 809–821 (2002).
Park, H. S. et al. Sequential activation of phosphatidylinositol 3-kinase, βPix, Rac1, and Nox1 in growth factor-induced production of H2O2 . Mol. Cell Biol. 24, 4384–4394 (2004).
Srinivasan, S. et al. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 160, 375–385 (2003).
Levi, S., Polyakov, M. V. & Egelhoff, T. T. Myosin II dynamics in Dictyostelium: determinants for filament assembly and translocation to the cell cortex during chemoattractant responses. Cell Motil. Cytoskeleton 53, 177–188 (2002).
Moores, S. L., Sabry, J. H. & Spudich, J. A. Myosin dynamics in live Dictyostelium cells. Proc. Natl Acad. Sci. USA 93, 443–446 (1996).
Eddy, R. J., Pierini, L. M., Matsumura, F. & Maxfield, F. R. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 113, 1287–1298 (2000).
Bosgraaf, L. et al. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. Embo J. 21, 4560–4570 (2002). Using mutants with deletions of guanylyl cyclases, cGMP-phosphodiesterases or cGMP-binding targets, the authors characterize the function of cGMP in D. discoideum as an inducer of myosin-II filaments in the cortex at the back of the cell, leading to suppression of lateral pseudopodia.
Liu, G. & Newell, P. C. Role of cyclic GMP in signal transduction to cytoskeletal myosin. Symp. Soc. Exp. Biol. 47, 283–295 (1993).
Goldberg, J. M., Bosgraaf, L., Van Haastert, P. J. M. & Smith, L. Identification of four candidate cGMP targets in Dictyostelium. Proc. Natl Acad. Sci. USA 99, 6749–6754 (2002).
Roelofs, J., Smith, J. L. & Van Haastert, P. J. M. cGMP signalling: different ways to create a pathway. Trends Genet. 19, 132–134 (2002).
Chung, C. Y., Potikyan, G. & Firtel, R. A. Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol. Cell 7, 937–947 (2001).
De La Roche, M. A., Smith, J. L., Betapudi, V., Egelhoff, T. T. & Cote, G. P. Signaling pathways regulating Dictyostelium myosin II. J. Muscle Res. Cell Motil. 23, 703–718 (2002).
Steimle, P. A. et al. Recruitment of a myosin heavy chain kinase to actin-rich protrusions in Dictyostelium. Curr. Biol. 11, 708–713 (2001).
Riento, K. & Ridley, A. J. ROCKs: multifunctional kinases in cell behaviour. Nature Rev. Mol. Cell Biol. 4, 446–456 (2003).
Van Haastert, P. J. M. & Van der Heijden, P. R. Excitation, adaptation, and deadaptation of the cAMP-mediated cGMP response in Dictyostelium discoideum. J. Cell Biol. 96, 347–353 (1983).
Berlot, C. H., Spudich, J. A. & Devreotes, P. N. Chemoattractant-elicited increases in myosin phosphorylation in Dictyostelium. Cell 43, 307–314 (1985).
Zhang, H. et al. Phosphorylation of the myosin regulatory light chain plays a role in motility and polarity during Dictyostelium chemotaxis. J. Cell Sci. 115, 1733–1747 (2002).
Stock, A. M. & Mowbray, S. L. Bacterial chemotaxis: a field in motion. Curr. Opin. Struct. Biol. 5, 744–751 (1995).
Caterina, M. J., Devreotes, P. N., Borleis, J. & Hereld, D. Agonist-induced loss of ligand binding is correlated with phosphorylation of cAR1, a G protein-coupled chemoattractant receptor from Dictyostelium. J. Biol. Chem. 270, 8667–8672 (1995).
Valkema, R. & Van Haastert, P. J. Inhibition of receptor-stimulated guanylyl cyclase by intracellular calcium ions in Dictyostelium cells. Biochem. Biophys. Res. Commun. 186, 263–268 (1992).
Kuwayama, H. & Van Haastert, P. J. Regulation of guanylyl cyclase by a cGMP-binding protein during chemotaxis in Dictyostelium discoideum. J. Biol. Chem. 271, 23718–23724 (1996).
Bosgraaf, L. et al. Identification and characterization of two unusual cGMP-stimulated phoshodiesterases in Dictyostelium. Mol. Biol. Cell 13, 3878–3889 (2002).
Valkema, R. & Van Haastert, P. J. M. A model for cAMP-mediated cGMP response in Dictyostelium discoideum. Mol. Biol. Cell 5, 575–585 (1994).
Jin, T., Zhang, N., Long, Y., Parent, C. A. & Devreotes, P. N. Localization of the G protein βγ complex in living cells during chemotaxis. Science 287, 1034–1036 (2000).
Sadhu, C., Masinovsky, B., Dick, K., Sowell, C. G. & Staunton, D. E. Essential role of phosphoinositide 3-kinase δ in neutrophil directional movement. J. Immunol. 170, 2647–2654 (2003).
Sulis, M. L. & Parsons, R. PTEN: from pathology to biology. Trends Cell Biol. 13, 478–483 (2003).
Traynor-Kaplan, A. E. et al. Transient increase in phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol trisphosphate during activation of human neutrophils. J. Biol. Chem. 264, 15668–15673 (1989).
Roos, W., Scheidegger, C. & Gerish, G. Adenylate cyclase activity oscillations as signals for cell aggregation in Dictyostelium discoideum. Nature 266, 259–261 (1977).
Jackowski, S. & Sha'afi, R. I. Response of adenosine cyclic 3′,5′-monophosphate level in rabbit neutrophils to the chemotactic peptide formyl-methionyl-leucyl-phenylalanine. Mol. Pharmacol. 16, 473–481 (1979).
van Haastert, P. J. & van Dijken, P. Biochemistry and genetics of inositol phosphate metabolism in Dictyostelium. FEBS Lett. 410, 39–43 (1997).
Berridge, M. J. & Irvine, R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312, 315–321 (1984).
Bumann, J., Wurster, B. & Malchow, D. Attractant-induced changes and oscillations of the extracellular Ca++ concentration in suspensions of differentiating Dictyostelium cells. J. Cell Biol. 98, 173–178 (1984).
Klein, P., Vaughan, R., Borleis, J. & Devreotes, P. The surface cyclic AMP receptor in Dictyostelium Levels of ligand-induced phosphorylation, solubilization, identification of primary transcript, and developmental regulation of expression. J. Biol. Chem. 262, 358–364 (1987).
Xiao, Z., Zhang, N., Murphy, D. B. & Devreotes, P. N. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J. Cell Biol. 139, 365–374 (1997).
Servant, G., Weiner, O. D., Neptune, E. R., Sedat, J. W. & Bourne, H. R. Dynamics of a chemoattractant receptor in living neutrophils during chemotaxis. Mol. Biol. Cell 10, 1163–1178 (1999).
Chen, L. et al. Two phases of actin polymerization display different dependencies on PI(3,4,5)P3 accumulation and have unique roles during chemotaxis. Mol. Biol. Cell. 14, 5028–5037 (2003).
Acknowledgements
The authors wish to thank all the members of their laboratories who have contributed much of the work discussed in this review. P.N.D. is supported by grants from the National Institutes of Health. P.V.H. is supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- NEUTROPHIL
-
A phagocytic cell of the myeloid lineage that has an important role in the inflammatory response. It undergoes chemotaxis towards sites of infection or wounding.
- FLAGELLUM
-
The cell-motility apparatus in swimming bacteria.
- LEADING EDGE
-
The thin margin of a lamellipodium that spans the area of the cell from the plasma membrane to about 1 μm back into the lamellipodium.
- UROPOD
-
A slender appendage that is formed at the trailing, rear edge of fast-migrating cells such as amoebae, neutrophils or lymphocytes.
- SEVEN-TRANSMEMBRANE-SPANNING RECEPTOR
-
A receptor that contains seven membrane-spanning helices and usually transmits signals to the inside of a cell by activating heterotrimeric G proteins.
- HETEROTRIMERIC G PROTEIN
-
A protein complex of three proteins (Gα, Gβ, and Gγ). Whereas Gβ and Gγ form a tight complex, Gα is part of the complex in its inactive, GDP-bound, form but dissociates in its active, GTP-bound, form. Both Gα and Gβγ can transmit downstream signals after activation.
- FRET
-
(fluorescence resonance energy transfer). A method to identify the proximity of two proteins, each of which is labelled with a different fluorescent group.
- PLECKSTRIN-HOMOLOGY (PH) DOMAIN
-
A sequence of 100 amino acids that is present in many signalling molecules and binds to lipid products of phosphatidylinositol 3-kinase. Pleckstrin is a protein of unknown function that was originally identified in platelets. It is a principal substrate of protein kinase C.
- Arp2/3 COMPLEX
-
A complex that consists of two actin-related proteins, Arp2 and Arp3, along with five smaller proteins. When activated, the Arp2/3 complex binds to the side of an existing actin filament and nucleates the assembly of a new actin filament. The resulting branch structure is Y-shaped.
- RHO-FAMILY GTPases
-
Ras-related small GTPases that are involved in controlling the polymerization of actin.
- GUANINE NUCLEOTIDE-EXCHANGE FACTOR (GEF)
-
A protein that facilitates the exchange of GDP for GTP in the nucleotide-binding pocket of a GTP-binding protein.
- DOMINANT-NEGATIVE
-
A defective protein that retains interaction capabilities and so distorts or competes with normal proteins.
Rights and permissions
About this article
Cite this article
Van Haastert, P., Devreotes, P. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol 5, 626–634 (2004). https://doi.org/10.1038/nrm1435
Issue Date:
DOI: https://doi.org/10.1038/nrm1435
This article is cited by
-
Tubulointerstitial nephritis antigen-like 1 is a novel matricellular protein that promotes gastric bacterial colonization and gastritis in the setting of Helicobacter pylori infection
Cellular & Molecular Immunology (2023)
-
The Impact of Phenotypic Heterogeneity on Chemotactic Self-Organisation
Bulletin of Mathematical Biology (2022)
-
Cellular inertia
Scientific Reports (2021)
-
Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products
Scientific Reports (2021)
-
Chemotaxis and swarming in differentiated HL-60 neutrophil-like cells
Scientific Reports (2021)