Key Points
-
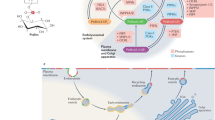
There is strong evidence for the presence of phospholipids inside the nucleus distinct from those that are in the nuclear envelope. The data indicate that these are not in a classic lipid bilayer, but their actual physicochemical form is not clearly understood.
-
Nuclei contain the enzymes necessary to make phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) from phosphatidylinositol, and they also contain at least one isoform of phosphoinositol lipid-specific phospholipase C (the β1 isoform). This can be activated by stimulation of cells with growth factors such as insulin-like growth factor 1 (IGF-1), and the resulting diacylglycerol (DAG) formation can recruit protein kinase C (PKC) to the nucleus.
-
There is also clear evidence for the activation of this PI-PLC pathway at different points of the normal cell cycle. In particular, at G2–M, a pulse of DAG generation from PtdIns(4,5)P2 causes the recruitment of PKCβII to the nucleus, in which its physiological function might be to phosphorylate lamins and thereby regulate nuclear envelope breakdown.
-
Another product of PI-PLC hydrolysis of PtdIns(4,5)P2, inositol-1,4,5-trisphosphate (Ins(1,4,5)P3), might regulate intranuclear calcium levels. It could also function as a precursor of more highly phosphorylated inositol phosphates, which have been suggested to be involved with other nuclear functions, such as messenger RNA export.
-
PtdIns(4,5)P2 might have other intranuclear roles — in particular, a proposed action in the regulation of RNA splicing.
-
Members of the phosphatidylinositol 3-kinase (PI3K) family, which synthesize 3-phosphorylated inositol lipids, have also been reported to be in the nucleus, although their intranuclear functions, if any, are not yet clearly defined.
Abstract
During the past twenty years, evidence has accumulated for the presence of phospholipids within the nuclei of eukaryotic cells. These phospholipids are distinct from those that are obviously present in the nuclear envelope. The best characterized of the intranuclear lipids are the inositol lipids that form the components of a phosphoinositide–phospholipase C cycle. However, exactly as has been discovered in the cytoplasm, this is just part of a complex picture that involves many other lipids and functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
D'Santos, C. S., Clarke, J. H. & Divecha, N. Phospholipid signalling in the nucleus. Een DAG uit het leven van de inositide signalering in de nucleus. Biochim. Biophys. Acta 1436, 201–232 (1998).
Cocco, L., Martelli, A. M., Gilmour, R. S., Rhee, S. G. & Manzoli, F. A. Nuclear phospholipase C and signaling. Biochim. Biophys. Acta 1530, 1–14 (2001).
Martelli, A. M. et al. Re-examination of the mechanisms regulating nuclear inositol lipid metabolism. FEBS Lett. 505, 1–6 (2001).
Irvine, R. F. Nuclear lipid signalling. STKE <http://stke.sciencemag.org/cgi/content/full/sigtrans;2002/150/re13> (2002).
Martelli, A. et al. Nuclear inositol lipid signaling and its potential involvement in malignant transformation. Biochim. Biophys. Acta 1603, 11–17 (2002).
Smith, C. D. & Wells, W. W. Phosphorylation of rat liver envelopes, characterisation of in vitro phosphorylation. J. Biol. Chem. 258, 765–770 (1983).
Cocco, L. et al. Synthesis of polyphosphoinositides in the nuclei of Friend cells. Biochem. J. 248, 765–770 (1987). This paper, together with references 9 and 10, provides the first evidence that nuclei contain a PtdIns(4,5)P 2 -based signalling system that is distinct from that in the cytoplasm.
Cocco, L. et al. Rapid changes in phospholipid metabolism in the nuclei of Swiss 3T3 cells induced by treatment of the cells with insulin like growth factor I. Biochem. Biophys. Res. 154, 1266–1272 (1988).
Divecha, N., Banfic, H. & Irvine, R. F. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-1) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J. 10, 3207–3214 (1991).
Martelli, A. M. et al. Temporal changes in intracellular distribution of protein kinase C in Swiss 3T3 cells during mitogenic stimulation with insulin-like growth factor I and bombesin: translocation to the nucleus follows rapid changes in nuclear polyphosphoinositides. Biochem. Biophys. Res. Commun. 177, 480–487 (1991).
Hinchliffe, K. A., Ciruela, A. & Irvine, R. F. PIPkins, their substrates and their products: new functions for old enzymes. Biochim. Biophys. Acta 1436, 87–104 (1998).
Vanhaesebroeck, B. et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70, 535–602 (2001).
Irvine, R. F. & Schell, M. J. Back in the water: the return of the inositol phosphates. Nature Rev. Mol. Cell Biol. 2, 327–338 (2001).
Vann, L. R., Wooding, F. B., Irvine, R. F. & Divecha, N. Metabolism and possible compartmentalization of inositol lipids in isolated rat-liver nuclei. Biochem. J. 327, 569–576 (1997).
Watt, S. A., Kular, G., Fleming, I. N., Downes, C. P. & Lucocq, J. M. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C-δ1. Biochem. J. 363, 657–666 (2002).
Jamney, P. A. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu. Rev. Physiol. 56, 169–191 (1994).
Cook, P. R. The organization of replication and transcription. Science 284, 1790–1795 (1999).
Rando, O. J., Zhao, K. & Crabtree, G. R. searching for a function for nuclear actin. Trends Cell Biol. 10, 92–97 (2000).
Osborne, S. L., Thomas, C. L., Gschmeissner, S. & Schiavo, G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J. Cell Sci. 114, 2501–2511 (2001). This paper provides the particularly intriguing suggestion for the involvement of PtdIns(4,5)P 2 in some aspect of pre-mRNA splicing.
Fricker, M., Hollinshead, M., White, N. & Vaux, D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J. Cell Biol. 136, 531–544 (1997).
Strouboulis, J. & Wolffe, A. P. Functional compartmentalization of the nucleus. J. Cell Sci. 109, 1991–2000 (1996).
DeLong, C. J., Qin, L. & Cui, Z. Nuclear localization of enzymatically active green fluorescent protein–CTP: phosphocholine cytidylyltransferase-α fusion protein is independent of cell cycle conditions and cell types. J. Biol. Chem. 275, 32325–32330 (2000).
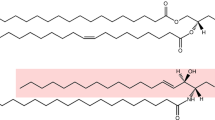
Hunt, A. N., Clark, G. T., Attard, G. S. & Postle, A. D. Highly saturated endonuclear phosphatidylcholine is synthesized in situ and colocated with CDP-choline pathway enzymes. J. Biol. Chem. 276, 8492–8499 (2001). By using mass-spectrometric analysis of deuterated species, this paper follows the distinct nuclear remodelling of PtdCho so that its fatty acyl chains are predominantly saturated. In this form it might have an important structural role.
Postle, A. D., Heeley, E. L. & Wilton, D. C. A comparison of the molecular species compositions of mammalian lung surfactant phospholipids. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129, 65–73 (2001).
Payrastre, B. et al. A Differential location of phosphoinositide kinases, diacylglycerol kinase, and phospholipase C in the nuclear matrix. J. Biol. Chem. 267, 5078–5084 (1992).
Boronenkov, I. V., Loijens, J. C., Umeda, M. & Anderson, R. A. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol. Biol. Cell. 9, 3547–3560 (1998).
Divecha, N., Treagus, J., Vann, L. & D'Santos, C. Phospholipases in the nucleus. Semin. Cell Dev. Biol. 8, 323–331 (1997).
Martelli, A. M. et al. Nuclear localisation and signalling activity of phosphoinositidase Cβ in Swiss 3T3 cells. Nature 358, 242–244 (1992).
Divecha, N., Rhee, S. G., Letcher, A. J. & Irvine, R. F. Phosphoinositide signalling enzymes in rat liver nuclei: phosphoinositidase C isoform β1 is specifically, but not predominately, located in the nucleus. Biochem. J. 289, 617–620 (1993).
Matteucci, A. et al. Nuclear but not cytoplasmic phospholipase C-β1 inhibits differentiation of erythroleukemia cells. Cancer Res. 58, 5057–5060 (1998).
Ye, K. et al. Phospholipase C-γ1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415, 541–544 (2002).
Rhee, S. G. & Bae, Y. S. Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 272, 15045–15048 (1997).
Faenza, I. et al. A role for nuclear phospholipase C-β1 in cell cycle control. J. Biol. Chem. 275, 30520–30524 (2000).
Bahk, Y. Y. et al. Two forms of phospholipase C-β1 generated by alternative splicing. J. Biol. Chem. 269, 8240–8245 (1994).
Kim, C. G., Park, D. & Rhee, S. G. The role of carboxyl-terminal basic amino acids in Gqα-dependent activation, particulate association, and nuclear localization of phospholipase C-β1. J. Biol. Chem. 271, 21187–21192 (1996).
Bahk, Y. Y. et al. Localization of two forms of phospholipase C-β1, a and b, in C6Bu-1 cells. Biochim. Biophys. Acta 1389, 76–80 (1998).
Crouch, M. F. & Simson, L. The G-protein Gi regulates mitosis but not DNA synthesis in growth factor-activated fibroblasts: a role for the nuclear translocation of Gi . FASEB J. 11, 189–198 (1997).
Martelli, A. M. et al. Insulin-like growth factor-I-dependent stimulation of nuclear phospholipase C-β1 activity in Swiss 3T3 cells requires an intact cytoskeleton and is paralleled by increased phosphorylation of the phospholipase. J. Cell. Biochem. 72, 339–348 (1999).
Martelli, A. M. et al. Insulin selectively stimulates nuclear phosphoinositide-specific phospholipase C (PI-PLC) β1 activity through a mitogen-activated protein (MAP) kinase-dependent serine phosphorylation. FEBS Lett. 486, 230–236 (2000).
Xu, A. et al. Phosphorylation of nuclear phospholipase C-β1 by extracellular signal-regulated kinase mediates the mitogenic action of insulin-like growth factor I. Mol. Cell Biol. 21, 2981–2990 (2001). This paper gives us a potentially important lead towards the problem of the intranuclear regulation of PI-PLCβ 1 — it is phosphorylated by extracellular signal-regulated protein kinases.
Manzoli, L. et al. Essential role for nuclear phospholipase C-β1 in insulin-like growth factor I-induced mitogenesis. Cancer Res. 57, 2137–2139 (1997).
Xu, A., Wang, Y., Xu, L. Y. & Gilmour, R. S. Protein kinase C-α-mediated negative feedback regulation is responsible for the termination of insulin-like growth factor I-induced activation of nuclear phospholipase C-β1 in Swiss 3T3 cells. J. Biol. Chem. 276, 14980–14986 (2001).
Divecha, N., Letcher, A. J., Banfic, H. H., Rhee, S. G. & Irvine, R. F. Changes in the components of a nuclear inositide cycle during differentiation in murine erythroleukaemia cells. Biochem. J. 312, 63–67 (1995).
Faenza, I. et al. Nuclear PLC-β1 acts as a negative regulator of p45/NF-E2 expression levels in Friend erythroleukemia cells. Biochim. Biophys. Acta 1589, 305–310 (2002).
Avazeri, N., Courtot, A. M., Pesty, A., Duquenne, C. & Lefevre, B. Cytoplasmic and nuclear phospholipase C-β1 relocation: role in resumption of meiosis in the mouse oocyte. Mol. Biol. Cell 11, 4369–4380 (2000).
Kim, D. et al. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature 389, 290–293 (1997).
Hutchison, C. J. Lamins: building blocks or regulators of gene expression? Nature Rev. Mol. Cell Biol. 3, 848–858 (2002).
Liu, N., Fukami, K., Yu, H. & Takenawa, T. A new phospholipase C-δ4 is induced at S-phase of the cell cycle and appears in the nucleus. J. Biol. Chem. 271, 355–360 (1996).
Lee, S. B. & Rhee, S. G. Molecular cloning, splice variants, expression, and purification of phospholipase C-δ4. J. Biol. Chem. 271, 25–31 (1996).
York, J. D., Odom, A. R., Murphy, R., Ives, E. B. & Wente, S. R. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science 295, 96–100 (1999).
Odom, A. R., Stahlberg, A., Wente, S. R. & York, J. D. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science 287, 2026–2029 (2000).
Shen, X., Xiao, H., Ranallo, R., Wu, W. -H. & Wu, C. Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates. Science 299, 112–114 (2003).
Steger, D. J., Haswell, E. S., Miller, A. L., Wente, S. R. & O'Shea, E. K. Regulation of chromatin remodeling by inositol polyphosphates. Science 299, 114–116 (2003). References 50–53 document another potentially important insight into the physiological function of the PI-PLC pathway in nuclei: to liberate Ins(1,4,5)P 3 , which is then phosphorylated to higher inositol phosphates. These in turn might have functions in mRNA export, transcriptional regulation and/or chromatin structure.
Yamaga, M., Fujii, M., Kamata, H., Hirata, H. & Yagisawa, H. Phospholipase C-δ1 contains a functional nuclear export signal sequence. J. Biol. Chem. 274, 28537–28541 (1999).
Huynh, C. V., Molecular genetic analysis of a phosphoinositide-specific phospholipase C (PLC1 gene product) in the yeast Saccharomyces cerevisiae. Thesis, Univ. California, Berkeley (1998).
Berridge, M. J. & Irvine, R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312, 315–321 (1984).
Leach, K. L. et al. α-thrombin stimulates nuclear diglyceride levels and differential nuclear localisation of protein kinase C isozymes in IIC9 Cells. J. Biol. Chem. 267, 21816–21822 (1992).
D'Santos, C. S., Clarke, J. H., Irvine, R. F. & Divecha, N. Nuclei contain two differentially regulated pools of diacylglycerol. Curr. Biol. 9, 437–440 (1999).
Deacon, E. M. et al. Generation of diacylglycerol molecular species through the cell cycle: a role for 1-stearoly, 2-arachidonoyl glycerol in the activation of nuclear protein kinase C-βII at G2/M. J. Cell Sci. 115, 983–989 (2002).
Sun, B., Murray, N. R. & Fields, A. P. A role for nuclear phosphatidylinositol-specific phospholipase C in the G2/M phase transition. J. Biol. Chem. 272, 26313–26317 (1997).
Neri, L. M., Borgatti, P., Capitani, S. & Martelli, A. M. Nuclear diacylglycerol produced by phosphoinositide-specific phospholipase C is responsible for nuclear translocation of protein kinase C-α. J. Biol. Chem. 273, 29738–29744 (1998).
Neri, L. M. et al. Proliferating or differentiating stimuli act on different lipid-dependent signaling pathways in nuclei of human leukemia cells. Mol. Biol. Cell 13, 947–964 (2002).
Banfic, B., Zizak, M., Divecha, N. & Irvine, R. F. Nuclear diacylglycerol is increased during cell proliferation in vivo. Biochem. J. 290, 633–636 (1993).
York, J. D. & Majerus, P. W. Nuclear phosphatidylinositols decrease during S-phase of the cell cycle in HeLa cells. J. Biol. Chem. 269, 7847–7850 (1994).
Irvine, R. F. & Divecha, N. Phospholipids in the nucleus — metabolism and possible functions. Semin. Cell Biol. 3, 225–235 (1992).
Fields, A. P. & Thompson, L. J. The regulation of mitotic nuclear envelope breakdown: a role for multiple lamin kinases. Prog. Cell Cycle Res. 1, 271–286 (1995).
Thompson, L. J. & Fields, A. P. βII protein kinase C is required for the G2/M phase transition of cell cycle. J. Biol. Chem. 271, 15045–15053 (1996). This is perhaps the crucial part of a series of papers (that includes references 70, 71 and 73), which builds up a convincing story for the generation of DAG from PtdIns(4,5)P 2 at G 2 –M. This in turn recruits PKCβII to the nucleus, where it phosphorylates lamins and participates in the regulation of nuclear-envelope breakdown.
Muranyi, W., Haas, J., Wagner, M., Krohne, G. & Koszinowski, U. H. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297, 854–857 (2002).
Chiarini, A., Whitfield, J. F., Armato, U. & Dal Pra, I. Protein kinase C-βII Is an apoptotic lamin kinase in polyomavirus-transformed, etoposide-treated pyF111 rat fibroblasts. J. Biol. Chem. 277, 18827–18839 (2002).
Hocevar, B. A. & Fields, A. P. Selective translocation of βII-protein kinase C to the nucleus of human promyelocytic (HL60) leukemia cells. J. Biol. Chem. 266, 28–33 (1991).
Fields, A. P., Tyler, G., Kraft, A. S. & May, W. S. Role of nuclear protein kinase C in the mitogenic response to platelet-derived growth factor. J. Cell Sci. 96, 107–114 (1990).
Neri, L. M. et al. Increase in nuclear phosphatidylinositol 3-kinase activity and phosphatidylinositol (3,4,5) trisphosphate synthesis precede PKC-ζ translocation to the nucleus of NGF-treated PC12 cells. FASEB J. 13, 2299–2310 (1999).
Goss, V. L. et al. Identification of nuclear βII protein kinase C as a mitotic lamin kinase. J. Biol. Chem. 269, 19074–19080 (1994).
Martelli, A. M. et al. Molecular characterization of protein kinase C-α binding to lamin A. J. Cell. Biochem. 86, 320–330 (2002).
Goto, K. & Kondo, H. Molecular cloning and expression of a 90-kDa diacylglycerol kinase that predominantly localizes in neurons. Proc. Natl Acad. Sci. USA 90, 7598–7602 (1993).
Zhao, K. et al. Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell 95, 625–636 (1998).
Yu, H., Fukami, K., Watanabe, Y., Ozaki, C. & Takenawa, T. Phosphatidylinositol 4,5-bisphosphate reverses the inhibition of RNA transcription caused by histone H1. Eur. J. Biochem. 251, 281–287 (1998).
Lu, P. J. et al. Phosphoinositide 3-kinase in rat liver nuclei. Biochemistry 37, 5738–5745 (1998).
Metjian, A., Roll, R. L., Ma, A. D. & Abrams, C. S. Agonists cause nuclear translocation of phosphatidylinositol 3-kinase-γ. A G β/γ-dependent pathway that requires the p110γ amino terminus. J. Biol. Chem. 274, 27943–27947 (1999).
Bacqueville, D. et al. Characterization of a G protein-activated phosphoinositide 3-kinase in vascular smooth muscle cell nuclei. J. Biol. Chem. 276, 22170–22176 (2001).
Lachyankar, M. B. et al. A role for nuclear PTEN in neuronal differentiation. J. Neurosci. 20, 1404–1413 (2000).
Gimm, O. et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am. J. Pathol. 156, 1693–1700 (2000).
Ye, K. et al. PIKE. A nuclear GTPase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell 103, 919–930 (2000).
Bae, S. S. et al. Src homology domains of phospholipase C-γ1 inhibit nerve growth factor-induced differentiation of PC12 cells. J. Neurochem. 71, 178–185 (1998).
Didichenko, S. A. & Thelen, M. Phosphatidylinositol 3-kinase c2α contains a nuclear localization sequence and associates with nuclear speckles. J. Biol. Chem. 276, 48135–48142 (2001).
Sindic, A., Aleksandrova, A., Fields, A. P., Volinia, S. & Banfic, H. Presence and activation of nuclear phosphoinositide 3-kinase C2β during compensatory liver growth. J. Biol. Chem. 276, 17754–17761 (2001).
Visnjic, D. et al. The activation of nuclear phosphoinositide 3-kinase C2β in all-trans-retinoic acid-differentiated HL-60 cells. FEBS Lett. 529, 268 (2002).
Yokogawa, T. et al. Evidence that 3′-phosphorylated polyphosphoinositides are generated at the nuclear surface: use of immunostaining technique with monoclonal antibodies specific for PI3,4-P2 . FEBS Lett. 473, 222–226 (2000).
Gillooly, D. J. et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19, 4577–4588 (2000).
Bunney, T. D. et al. Association of phosphatidylinositol 3-kinase with nuclear transcription sites in higher plants. Plant Cell 12, 1679–1688 (2000).
Tanaka, K. et al. Evidence that a phosphatidylinositol 3,4,5-trisphosphate-binding protein can function in nucleus. J. Biol. Chem. 274, 3919–3922 (1999).
Neri, L. M., Borgatti, P., Capitani, S. & Martelli, A. M. The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim. Biophys. Acta 1584, 73 (2002).
Glover, S. et al. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J. Biol. Chem. 270, 15359–15367 (1995).
Schievella, A. R., Regier, M. K., Smith, W. L. & Lin, L. L. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J. Biol. Chem. 270, 30749–30754 (1995).
Peters-Golden, M., Song, K., Marshall, T. & Brock, T. Translocation of cytosolic phospholipase A2 to the nuclear envelope elicits topographically localized phospholipid hydrolysis. Biochem. J. 318, 797–803 (1996).
Bhattacharya, M. et al. Nuclear localization of prostaglandin E2 receptors. Proc. Natl Acad. Sci. USA 95, 15792–15797 (1998).
Tamiya-Koizumi, K., Umekawa, H., Yoshida, S., Ishihara, H. & Kojima, K. A novel phospholipase A2 associated with nuclear matrix: stimulation of the activity and modulation of the Ca2+ dependency by polyphosphoinositides. Biochim. Biophys. Acta 1002, 182–188 (1989).
Fayard, J. M., Tessier, C., Pageaux, J. F., Lagarde, M. & Laugier, C. Nuclear location of PLA2-I in proliferative cells. J. Cell Sci. 111, 985–994 (1998).
Brock, T. G., Maydanski, E., McNish, R. W. & Peters-Golden, M. Co-localization of leukotriene A4 hydrolase with 5-lipoxygenase in nuclei of alveolar macrophages and rat basophilic leukemia cells but not neutrophils. J. Biol. Chem. 276, 35071–35077 (2001).
Funk, C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 (2001).
McIntyre, T. M. et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARγ agonist. Proc. Natl Acad. Sci. USA 100, 131–136 (2003).
Jarpe, M. B., Leach, K. L. & Raben, D. M. α-thrombin-induced nuclear sn-1,2-diacylglycerols are derived from phosphatidylcholine hydrolysis in cultured fibroblasts. Biochemistry 33, 526–534 (1994).
Murray, N. R. & Fields, A. P. Phosphatidylglycerol is a physiologic activator of nuclear protein kinase C. J. Biol. Chem. 273, 11514–11520 (1998).
Gokmen-Polar, Y. & Fields, A. P. Mapping of a molecular determinant for protein kinase C βII isozyme function. J. Biol. Chem. 273, 20261–20266 (1998).
Cocco, L., Maraldi, N. M., Manzoli, F. A., Gilmour, R. S. & Lang, A. Phospholipid interactions in rat liver nuclear matrix. Biochem. Biophys. Res. Commun. 96, 890–898 (1980).
Tamiya-Koizumi, K., Umekawa, H., Yoshida, S. & Kojima, K. Existence of Mg2+-dependent, neutral sphingomyelinase in nuclei of rat ascites hepatoma cells. J. Biochem. 106, 593–598 (1989).
Mizutani, Y. et al. Nuclear localization of neutral sphingomyelinase 1: biochemical and immunocytochemical analyses. J. Cell Sci. 114, 3727–3736 (2001).
Tsugane, K., Tamiya-Koizumi, K., Nagino, M., Nimura, Y. & Yoshida, S. A possible role of nuclear ceramide and sphingosine in hepatocyte apoptosis in rat liver. J. Hepatol. 31, 8–17 (1999).
Simbulan, C. M. et al. Sphingosine inhibits the synthesis of RNA primers by primase in vitro. Biochemistry 33, 9007–9012 (1994).
De Vries, K. J. et al. Fluorescently labeled phosphatidylinositol transfer protein isoforms (α and β), microinjected into fetal bovine heart endothelial cells, are targeted to distinct intracellular sites. Exp. Cell Res. 227, 33–39 (1996).
Rubbini, S. et al. Phosphoinositide signalling in nuclei of Friend cells: DMSO-induced differentiation reduces the association of phosphatidylinositol-transfer protein with the nucleus. Biochem. Biophys. Res. Commun. 230, 302–305 (1997).
Stephens, L. R., Hughes, K. T. & Irvine, R. F. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature 351, 33–39 (1991).
Rameh, L. E., Tolias, K. F., Duckworth, B. C. & Cantley, L. C. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 390, 192–196 (1997).
Sbrissa, D., Ikonomov, O. C., Deeb, R. & Shisheva, A. Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J. Biol. Chem. 277, 47276–47284 (2002).
Ciruela, A., Hinchliffe, K. A., Divecha, N. & Irvine, R. F. Nuclear targeting of the β isoform of Type II phosphatidylinositol phosphate kinase (phosphatidylinositol 5-phosphate 4-kinase) by its α-helix 7. Biochem. J. 346, 587–591 (2000).
Clarke, J. H. et al. Inositol lipids are regulated during cell cycle progression in the nuclei of murine erythroleukaemia cells. Biochem J. 357, 905–910 (2001).
de Graaf, P. et al. Nuclear localization of phosphatidylinositol 4-kinase-β. J. Cell Sci. 115, 1769–1775 (2002).
Hardingham, G. E., Cruzalegui, F. H., Chawla, S. & Bading, H. Mechanisms controlling gene expression by nuclear calcium signals. Cell Calcium 23, 131–134 (1998).
Malviya, A. N. & Rogue, P. J. “Tell me where is calcium bred?”: clarifying the roles of nuclear calcium. Cell 92, 17–23 (1998).
Bootman, M. D., Thomas, D., Tovey, S. C., Berridge, M. J. & Lipp, P. Nuclear calcium signalling. Cell. Mol. Life Sci. 57, 371–388 (2000).
Gerasimenko, O. V., Gerasimenko, J. V., Tepikin, A. V. & Petersen, O. H. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell 80, 439–444 (1995).
Adebanjo, O. A. et al. Novel biochemical and functional insights into nuclear Ca2+ transport through IP(3)Rs and RyRs in osteoblasts. Am. J. Physiol. Renal Physiol. 278, 784–791 (2000).
Hennager, D. J., Welsh, M. J. & DeLisle, S. Changes in either cytosolic or nucleoplasmic inositol 1,4,5-trisphosphate levels can control nuclear Ca2+ concentration. J. Biol. Chem. 270, 4959–4962 (1995).
Santella, L. & Kyozuka, K. Effects of 1-methyladenine on nuclear Ca2+ transients and meiosis resumption in starfish oocytes are mimicked by the nuclear injection of inositol 1,4,5-trisphosphate and cADP-ribose. Cell Calcium 22, 11–20 (1997).
Santella, L., De Riso, L., Gragnaniello, G. & Kyozuka, K. Separate activation of the cytoplasmic and nuclear calcium pools in maturing starfish. Biochem. Biophys. Res. Commun. 252, 1–4 (1998).
Shirakawa, H. & Miyazaki, S. Spatiotemporal analysis of calcium dynamics in the nucleus of hamster oocytes. J. Physiol. 494, 29–40 (1996).
Lipp, P., Thomas, D., Berridge, M. J. & Bootman, M. D. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J. 16, 7166–7173 (1997).
Topham, M. K. et al. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-ζ. Nature 394, 697–700 (1998). This paper describes an interesting regulation of the nuclear localization of a DAG kinase (the ζ-isoform), which attenuates a DAG signal by phosphorylating it to phosphatidic acid. DAG kinase-ζ is phosphorylated by PKC, and this leads to its exclusion from the nucleus.
Martelli, A. M. et al. Enhanced nuclear diacylglycerol kinase activity in response to a mitogenic stimulation of quiescent Swiss 3T3 cells with insulin-like growth factor I. Cancer Res. 60, 815–821 (2000).
Wada, I., Kai, M., Imai, S., Sakane, F. & Kanoh, H. Translocation of diacylglycerol kinase-α to the nuclear matrix of rat thymocytes and peripheral T-lymphocytes. FEBS Lett. 393, 48–52 (1996).
Bregoli, L., Baldassare, J. J. & Raben, D. M. Nuclear diacylglycerol kinase-θ is activated in response to α-thrombin. J. Biol. Chem. 276, 23288–23295 (2001).
Martelli, A. M. et al. Diacylglycerol kinases in nuclear lipid-dependent signal transduction pathways. Cell. Mol. Life Sci. 59, 1129–1137 (2002).
Burke, B. & Ellenberg, J. Remodelling the walls of the nucleus. Nature Rev. Mol. Cell Biol. 3, 487–497 (2002).
Pollard, T. D. & Earnshaw, W. C. Cell Biology (Elsevier, USA, 2002).
Halstead, J. R. et al. A novel pathway of cellular phosphatidylinositol(3,4,5)-trisphosphate synthesis is regulated by oxidative stress. Curr. Biol. 11, 386–395 (2001).
Acknowledgements
I am grateful to many colleagues, especially those in Cambridge, Amsterdam and Bologna, for helpful discussions and their suggestions, and to the Royal Society for its support.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
InterPro
LocusLink
Swiss-Prot
diacylglycerol cholinephosphotransferase
Glossary
- BOMBESIN
-
A strong releaser of gastrin and cholecystokinin that is found in the gut and the brain. It also has a mitogenic action in several cell types.
- PLECKSTRIN HOMOLOGY (PH) DOMAIN
-
A sequence of 100 amino acids that is present in many signalling molecules; some PH domains bind to lipid products of phosphoinositide 3-kinase. Pleckstrin is a protein of unknown function that was identified originally in platelets. It is a principal substrate of protein kinase C.
- SPLICEOSOME
-
A protein–U small nuclear RNA complex that is required for folding of the pre-messenger RNA into the correct conformation for the removal of introns.
- SRC-HOMOLOGY-3 (SH3) DOMAIN
-
A protein sequence of about 50 amino acids that recognizes and binds sequences that are rich in proline.
- DOMINANT-NEGATIVE
-
A defective protein that retains interaction abilities and so distorts or competes with its normal protein counterparts.
- CHROMATIN REMODELLING
-
Dynamic changes of chromatin organization, which are required for optimal execution of processes such as DNA replication, gene transcription, DNA repair or chromosome segregation.
- EF-HAND
-
A graphical description for the structure of a Ca2+-binding motif that was first described in parvalbumin.
- HELA CELLS
-
An established tissue-culture strain of human epidermoid carcinoma cells that contain 70–80 chromosomes per cell. These cells were derived originally from tissue that was taken from a patient named Henrietta Lacks in 1951.
- NOCODAZOLE BLOCK
-
An inhibition of cell-cycle progression that is caused by the effects of the nocodazole microtubule-depolymerizing drug on spindle assembly.
- ELUTRIATION
-
A centrifugal technique (which uses a special elutriation rotor) that separates cells into fractions that are dependent on their size. It can therefore be used to separate cells at different stages of the cell cycle from an asynchronous population.
- PHORBOL ESTER
-
A polycyclic ester that is isolated from croton oil. The most common is phorbol myristol acetate (PMA, also known as 12,13-tetradecanoyl phorbol acetate or TPA). Phorbol esters mimic diacylglycerol, and thereby activate protein kinase C.
- BRYOSTATIN
-
A highly potent activator of protein kinase C that is found in bryozoans.
- C2 DOMAIN
-
(conserved region 2 of protein kinase C). C2 domains are ∼100 amino-acids long and bind phospholipids and Ca2+ interdependently. They are present in many proteins that are involved in Ca2+ signalling.
- PC12 CELLS
-
A clonal line of rat adrenal pheochromocytoma cells that respond to nerve growth factor and can synthesize, store and secrete catecholamines, much like sympathetic neurons. PC12 cells contain small, clear synaptic-like vesicles and larger, dense core granules.
- GUANINE NUCLEOTIDE EXCHANGE FACTOR
-
A protein that facilitates the exchange of GDP (guanine diphosphate) for GTP (guanine triphosphate) in the nucleotide-binding pocket of a GTP-binding protein.
Rights and permissions
About this article
Cite this article
Irvine, R. Nuclear lipid signalling. Nat Rev Mol Cell Biol 4, 349–361 (2003). https://doi.org/10.1038/nrm1100
Issue Date:
DOI: https://doi.org/10.1038/nrm1100
This article is cited by
-
Confirmation of sub-cellular resolution using oversampling imaging mass spectrometry
Analytical and Bioanalytical Chemistry (2019)
-
Lipids in the cell: organisation regulates function
Cellular and Molecular Life Sciences (2018)
-
DNA damage causes rapid accumulation of phosphoinositides for ATR signaling
Nature Communications (2017)
-
The Ebola Virus matrix protein, VP40, requires phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) for extensive oligomerization at the plasma membrane and viral egress
Scientific Reports (2016)
-
Ezrin-related Phosphoinositide pathway modifies RhoA and Rac1 in human osteosarcoma cell lines
Journal of Cell Communication and Signaling (2015)