Key Points
-
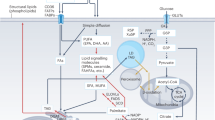
This Review focuses on the pathways that catabolize cellular triacylglycerol ('fat'), namely, neutral lipolysis, acid lipolysis and lipophagy.
-
Neutral lipolysis of triglycerides in cytosolic lipid droplets relies on three lipid hydrolases (lipases): adipose triglyceride lipase, hormone-sensitive lipase and monoacylglycerol lipase. The consecutive action of these enzymes provides free fatty acids and glycerol for energy production and other metabolic pathways during fasting. The regulation of neutral lipolysis is complex and involves numerous proteins, hormones, growth factors and cytokines. Conversely, products and intermediates of neutral lipolysis regulate key metabolic pathways by transcriptional and post-transcriptional mechanisms.
-
Lipophagy is a subtype of macroautophagy. Portions of cytosolic lipid droplets are engulfed by lipoautophagosomes and transported to lysosomes, where triacylglycerols and other lipids undergo acid lipolysis by lysosomal acid lipase.
-
The regulation of acid lipolysis is less complex than the regulation of neutral lipolysis, but again, the products and intermediates of triacylglycerol hydrolysis exit lysosomes and regulate multiple key processes in energy metabolism.
-
Neutral lipolysis, acid lipolysis and lipophagy cooperate mechanistically.
-
Rare mutations in the genes coding for adipose triglyceride lipase; its co-activator, lipid droplet-binding protein CGI-58; hormone-sensitive lipase; and lysosomal acid lipase cause distinct metabolic disorders in humans.
-
Recent insights led to a better understanding of how cellular triacylglycerol catabolism affects the pathogenesis of metabolic diseases, cancer and cancer-associated cachexia and highlighted potential treatment strategies for lipid-associated disorders.
Abstract
Fatty acids are the most efficient substrates for energy production in vertebrates and are essential components of the lipids that form biological membranes. Synthesis of triacylglycerols from non-esterified free fatty acids (FFAs) combined with triacylglycerol storage represents a highly efficient strategy to stockpile FFAs in cells and prevent FFA-induced lipotoxicity. Although essentially all vertebrate cells have some capacity to store and utilize triacylglycerols, white adipose tissue is by far the largest triacylglycerol depot and is uniquely able to supply FFAs to other tissues. The release of FFAs from triacylglycerols requires their enzymatic hydrolysis by a process called lipolysis. Recent discoveries thoroughly altered and extended our understanding of lipolysis. This Review discusses how cytosolic 'neutral' lipolysis and lipophagy, which utilizes 'acid' lipolysis in lysosomes, degrade cellular triacylglycerols as well as how these pathways communicate, how they affect lipid metabolism and energy homeostasis and how their dysfunction affects the pathogenesis of metabolic diseases. Answers to these questions will likely uncover novel strategies for the treatment of prevalent metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Unger, R. H. Lipotoxic diseases. Annu. Rev. Med. 53, 319–336 (2002).
Armand, M. Lipases and lipolysis in the human digestive tract: where do we stand? Curr. Opin. Clin. Nutr. Metab. Care 10, 156–164 (2007).
Young, S. G. & Zechner, R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 27, 459–484 (2013).
Zechner, R. FAT FLUX: enzymes, regulators, and pathophysiology of intracellular lipolysis. EMBO Mol. Med. 7, 359–362 (2015).
Dubland, J. A. & Francis, G. A. Lysosomal acid lipase: at the crossroads of normal and atherogenic cholesterol metabolism. Front. Cell Dev. Biol. 3, 3 (2015).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Zimmermann, R. et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 (2004).
Villena, J. A., Roy, S., Sarkadi-Nagy, E., Kim, K. H. & Sul, H. S. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279, 47066–47075 (2004).
Jenkins, C. M. et al. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279, 48968–48975 (2004).
Haemmerle, G. et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312, 734–737 (2006).
Vaughan, M., Berger, J. E. & Steinberg, D. Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. J. Biol. Chem. 239, 401–409 (1964).
Lass, A. et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3, 309–319 (2006).
Yang, X. et al. The G0/G1 switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 11, 194–205 (2010).
Radner, F. P. et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58). J. Biol. Chem. 285, 7300–7311 (2010).
Grond, S. et al. Skin barrier development depends on CGI-58 protein expression during late-stage keratinocyte differentiation. J. Invest. Dermatol. 137, 403–413 (2017).
Grond, S. et al. PNPLA1 deficiency in mice and humans leads to a defect in the synthesis of omega-O-Acylceramides. J. Invest. Dermatol. 137, 394–402 (2017).
Stone, S. J. et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 279, 11767–11776 (2004).
Russell, L. & Forsdyke, D. R. A human putative lymphocyte G0/G1 switch gene containing a CpG-rich island encodes a small basic protein with the potential to be phosphorylated. DNA Cell Biol. 10, 581–591 (1991).
Cerk, I. K. et al. A peptide derived from G0/G1 switch gene 2 acts as noncompetitive inhibitor of adipose triglyceride lipase. J. Biol. Chem. 289, 32559–32570 (2014).
Lu, X., Yang, X. & Liu, J. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle 9, 2719–2725 (2010).
Heckmann, B. L. et al. Defective adipose lipolysis and altered global energy metabolism in mice with adipose overexpression of the lipolytic inhibitor G0/G1 switch gene 2 (G0S2). J. Biol. Chem. 289, 1905–1916 (2014).
Zhang, X. et al. Targeted disruption of G0/G1 switch gene 2 enhances adipose lipolysis, alters hepatic energy balance, and alleviates high-fat diet-induced liver steatosis. Diabetes 63, 934–946 (2014).
Wang, Y. et al. The G0/G1 switch gene 2 is an important regulator of hepatic triglyceride metabolism. PLoS ONE 8, e72315 (2013).
Heier, C. et al. G0/G1 switch gene 2 regulates cardiac lipolysis. J. Biol. Chem. 290, 26141–26150 (2015).
Hofer, P. et al. Fatty acid-binding proteins interact with comparative gene identification-58 linking lipolysis with lipid ligand shuttling. J. Biol. Chem. 290, 18438–18453 (2015).
Notari, L. et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J. Biol. Chem. 281, 38022–38037 (2006).
Chung, C. et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL). J. Hepatol. 48, 471–478 (2008).
Borg, M. L. et al. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes 60, 1458–1466 (2011).
Singh, M. et al. Fat-specific protein 27 inhibits lipolysis by facilitating the inhibitory effect of transcription factor Egr1 on transcription of adipose triglyceride lipase. J. Biol. Chem. 289, 14481–14487 (2014).
Grahn, T. H. et al. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J. Biol. Chem. 289, 12029–12039 (2014).
Eichmann, T. O. et al. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem. 287, 41446–41457 (2012).
Rodriguez, J. A. et al. In vitro stereoselective hydrolysis of diacylglycerols by hormone-sensitive lipase. Biochim. Biophys. Acta 1801, 77–83 (2010).
Lafontan, M. & Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 48, 275–297 (2009).
Fischer, J. et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 39, 28–30 (2007).
Osuga, J. et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc. Natl Acad. Sci. USA 97, 787–792 (2000).
Albert, J. S. et al. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N. Engl. J. Med. 370, 2307–2315 (2014).
Petrosino, S. & Di Marzo, V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr. Opin. Investig. Drugs 11, 51–62 (2010).
Subramanian, V. et al. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J. Biol. Chem. 279, 42062–42071 (2004).
Granneman, J. G. & Moore, H. P. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol. Metab. 19, 3–9 (2008).
Watt, M. J. & Steinberg, G. R. Regulation and function of triacylglycerol lipases in cellular metabolism. Biochem. J. 414, 313–325 (2008).
Collins, S. A heart-adipose tissue connection in the regulation of energy metabolism. Nat. Rev. Endocrinol. 10, 157–163 (2014).
Kaltenecker, D. et al. Adipocyte STAT5 deficiency promotes adiposity and impairs lipid mobilisation in mice. Diabetologia 60, 296–330 (2016).
Saltiel, A. R. Insulin signaling in the control of glucose and lipid homeostasis. Handb Exp. Pharmacol. 233, 51–71 (2016).
DiPilato, L. M. et al. The role of PDE3B phosphorylation in the inhibition of lipolysis by insulin. Mol. Cell. Biol. 35, 2752–2760 (2015).
Chakrabarti, P. & Kandror, K. V. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J. Biol. Chem. 284, 13296–13300 (2009).
Chakrabarti, P. et al. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J. Lipid Res. 52, 1693–1701 (2011).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429, 771–776 (2004).
Lasa, A. et al. Resveratrol regulates lipolysis via adipose triglyceride lipase. J. Nutr. Biochem. 23, 379–384 (2012).
Lamming, D. W. & Sabatini, D. M. A. Central role for mTOR in lipid homeostasis. Cell Metab. 18, 465–469 (2013).
Ceddia, R. B. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol. Cell Endocrinol. 366, 194–203 (2013).
Chakrabarti, P., English, T., Shi, J., Smas, C. M. & Kandror, K. V. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes 59, 775–781 (2010).
Chakrabarti, P. et al. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway. Mol. Cell. Biol. 33, 3659–3666 (2013).
Kumar, A. et al. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes 59, 1397–1406 (2010).
Gaidhu, M. P. et al. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J. Lipid Res. 50, 704–715 (2009).
Kim, S. J. et al. AMPK phosphorylates desnutrin/ATGL and hormone-sensitive lipase to regulate lipolysis and fatty acid oxidation within adipose tissue. Mol. Cell. Biol. 36, 1961–1976 (2016).
Watt, M. J. et al. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 290, E500–E508 (2006).
Ahmadian, M. et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13, 739–748 (2011).
Pagnon, J. et al. Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology 153, 4278–4289 (2012).
Karlsson, M. et al. Exon-intron organization and chromosomal localization of the mouse monoglyceride lipase gene. Gene 272, 11–18 (2001).
Rakhshandehroo, M. et al. Comprehensive analysis of PPARα-dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Res. 2007, 26839 (2007).
Haemmerle, G. et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 17, 1076–1085 (2011).
Zierler, K. A. et al. Functional cardiac lipolysis in mice critically depends on comparative gene identification-58. J. Biol. Chem. 288, 9892–9904 (2013).
Schoiswohl, G. et al. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J. Lipid Res. 51, 490–499 (2010).
Wu, J. W. et al. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology 54, 122–132 (2011).
Ong, K. T., Mashek, M. T., Bu, S. Y., Greenberg, A. S. & Mashek, D. G. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology 53, 116–126 (2011).
Obrowsky, S. et al. Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARα signaling. J. Lipid Res. 54, 425–435 (2013).
Chandak, P. G. et al. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 285, 20192–20201 (2010).
Mottillo, E. P., Bloch, A. E., Leff, T. & Granneman, J. G. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) α and δ in brown adipocytes to match fatty acid oxidation with supply. J. Biol. Chem. 287, 25038–25048 (2012).
Schoiswohl, G. et al. Impact of reduced ATGL-mediated adipocyte lipolysis on obesity-associated insulin resistance and inflammation in male mice. Endocrinology 156, 3610–3624 (2015).
Jaeger, D. et al. Fasting-induced G0/G1 switch gene 2 and FGF21 expression in the liver are under regulation of adipose tissue derived fatty acids. J. Hepatol. 63, 437–445 (2015).
Tang, T. et al. Desnutrin/ATGL activates PPARδ to promote mitochondrial function for insulin secretion in islet beta cells. Cell Metab. 18, 883–895 (2013).
Schreiber, R. et al. Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesity. Proc. Natl Acad. Sci. USA 112, 13850–13855 (2015).
Haemmerle, G. et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 277, 4806–4815 (2002).
Armstrong, E. H., Goswami, D., Griffin, P. R., Noy, N. & Ortlund, E. A. Structural basis for ligand regulation of the fatty acid-binding protein 5, peroxisome proliferator-activated receptor beta/delta (FABP5-PPARβ/δ) signaling pathway. J. Biol. Chem. 289, 14941–14954 (2014).
Tan, N. S. et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol. Cell. Biol. 22, 5114–5127 (2002).
Wolfrum, C., Borrmann, C. M., Borchers, T. & Spener, F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors α- and γ-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc. Natl Acad. Sci. USA 98, 2323–2328 (2001).
Shen, W. J., Sridhar, K., Bernlohr, D. A. & Kraemer, F. B. Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proc. Natl Acad. Sci. USA 96, 5528–5532 (1999).
Smith, A. J. et al. Physical association between the adipocyte fatty acid-binding protein and hormone-sensitive lipase: a fluorescence resonance energy transfer analysis. J. Biol. Chem. 279, 52399–52405 (2004).
Khan, S. A. et al. ATGL-catalyzed lipolysis regulates SIRT1 to control PGC-1γ/PPAR-α signaling. Diabetes 64, 418–426 (2015).
Ng, F. & Tang, B. L. Sirtuins' modulation of autophagy. J. Cell. Physiol. 228, 2262–2270 (2013).
Lettieri Barbato, D., Tatulli, G., Aquilano, K. & Ciriolo, M. R. FoxO1 controls lysosomal acid lipase in adipocytes: implication of lipophagy during nutrient restriction and metformin treatment. Cell Death Dis. 4, e861 (2013).
Wagner, G. R. & Payne, R. M. Widespread and enzyme-independent Nepsilon-acetylation and Nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J. Biol. Chem. 288, 29036–29045 (2013).
Weinert, B. T. et al. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol. Syst. Biol. 10, 716 (2014).
Weinert, B. T., Moustafa, T., Iesmantavicius, V., Zechner, R. & Choudhary, C. Analysis of acetylation stoichiometry suggests that SIRT3 repairs nonenzymatic acetylation lesions. EMBO J. 34, 2620–2632 (2015).
Eisenberg, T. et al. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme a stimulates autophagy and prolongs lifespan. Cell Metab. 19, 431–444 (2014).
Marino, G. et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol. Cell 53, 710–725 (2014).
Pougovkina, O. et al. Mitochondrial protein acetylation is driven by acetyl-CoA from fatty acid oxidation. Hum. Mol. Genet. 23, 3513–3522 (2014).
Hebert, A. S. et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 49, 186–199 (2013).
Hirschey, M. D. et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 (2010).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Sheriff, S., Du, H. & Grabowski, G. A. Characterization of lysosomal acid lipase by site-directed mutagenesis and heterologous expression. J. Biol. Chem. 270, 27766–27772 (1995).
Warner, T. G., Dambach, L. M., Shin, J. H. & O'Brien, J. S. Purification of the lysosomal acid lipase from human liver and its role in lysosomal lipid hydrolysis. J. Biol. Chem. 256, 2952–2957 (1981).
Grumet, L. et al. Lysosomal acid lipase hydrolyzes retinyl ester and affects retinoid turnover. J. Biol. Chem. 291, 17977–17987 (2016).
Sando, G. N. & Henke, V. L. Recognition and receptor-mediated endocytosis of the lysosomal acid lipase secreted by cultured human fibroblasts. J. Lipid Res. 23, 114–123 (1982).
Haka, A. S. et al. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol. Biol. Cell 20, 4932–4940 (2009).
Goldstein, J. L. & Brown, M. S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172 (2015).
Dieckmann, M., Dietrich, M. F. & Herz, J. Lipoprotein receptors—an evolutionarily ancient multifunctional receptor family. Biol. Chem. 391, 1341–1363 (2010).
Du, H., Duanmu, M., Witte, D. & Grabowski, G. A. Targeted disruption of the mouse lysosomal acid lipase gene: long-term survival with massive cholesteryl ester and triglyceride storage. Hum. Mol. Genet. 7, 1347–1354 (1998).
Du, H. et al. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J. Lipid Res. 42, 489–500 (2001).
Radovic, B. et al. Lysosomal acid lipase regulates VLDL synthesis and insulin sensitivity in mice. Diabetologia 59, 1743–1752 (2016).
Settembre, C. et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 15, 647–658 (2013).
Martina, J. A. et al. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal 7, ra9 (2014).
Raben, N. & Puertollano, R. TFEB and TFE3: linking lysosomes to cellular adaptation to stress. Annu. Rev. Cell Dev. Biol. 32, 255–278 (2016).
O'Rourke, E. J. & Ruvkun, G. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat. Cell Biol. 15, 668–676 (2013).
Emanuel, R. et al. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler. Thromb. Vasc. Biol. 34, 1942–1952 (2014).
Cox, B. E., Griffin, E. E., Ullery, J. C. & Jerome, W. G. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidification. J. Lipid Res. 48, 1012–1021 (2007).
Li, W., Yuan, X. M., Olsson, A. G. & Brunk, U. T. Uptake of oxidized LDL by macrophages results in partial lysosomal enzyme inactivation and relocation. Arterioscler Thromb. Vasc. Biol. 18, 177–184 (1998).
Griffin, E. E., Ullery, J. C., Cox, B. E. & Jerome, W. G. Aggregated LDL and lipid dispersions induce lysosomal cholesteryl ester accumulation in macrophage foam cells. J. Lipid Res. 46, 2052–2060 (2005).
Bowden, K. L. et al. Lysosomal acid lipase deficiency impairs regulation of ABCA1 gene and formation of high density lipoproteins in cholesteryl ester storage disease. J. Biol. Chem. 286, 30624–30635 (2011).
Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 125–138 (2008).
Li, J. & Pfeffer, S. R. Lysosomal membrane glycoproteins bind cholesterol and contribute to lysosomal cholesterol export. eLife 5, e21635 (2016).
Chen, F. W., Gordon, R. E. & Ioannou, Y. A. NPC1 late endosomes contain elevated levels of non-esterified ('free') fatty acids and an abnormally glycosylated form of the NPC2 protein. Biochem. J. 390, 549–561 (2005).
Passeggio, J. & Liscum, L. Flux of fatty acids through NPC1 lysosomes. J. Biol. Chem. 280, 10333–10339 (2005).
Lee, S. D. & Tontonoz, P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis 242, 29–36 (2015).
Folick, A. et al. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science 347, 83–86 (2015).
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
Lamb, C. A., Yoshimori, T. & Tooze, S. A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 14, 759–774 (2013).
Efeyan, A., Comb, W. C. & Sabatini, D. M. Nutrient-sensing mechanisms and pathways. Nature 517, 302–310 (2015).
Feng, Y., Yao, Z. & Klionsky, D. J. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 25, 354–363 (2015).
Lee, J. M. et al. Nutrient-sensing nuclear receptors coordinate autophagy. Nature 516, 112–115 (2014).
Ao, X., Zou, L. & Wu, Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 21, 348–358 (2014).
Schroeder, B. et al. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology 61, 1896–1907 (2015).
Itakura, E., Kishi-Itakura, C. & Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151, 1256–1269 (2012).
Saftig, P., Beertsen, W. & Eskelinen, E. L. LAMP-2: a control step for phagosome and autophagosome maturation. Autophagy 4, 510–512 (2008).
Mijaljica, D., Prescott, M. & Devenish, R. J. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy 7, 673–682 (2011).
Cingolani, F. & Czaja, M. J. Regulation and functions of autophagic lipolysis. Trends Endocrinol. Metab. 27, 696–705 (2016).
Komatsu, M. et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434 (2005).
Shibata, M. et al. The MAP1-LC3 conjugation system is involved in lipid droplet formation. Biochem. Biophys. Res. Commun. 382, 419–423 (2009).
Conlon, D. M. et al. Inhibition of apolipoprotein B synthesis stimulates endoplasmic reticulum autophagy that prevents steatosis. J. Clin. Invest. 126, 3852–3867 (2016).
Kwanten, W. J. et al. Hepatocellular autophagy modulates the unfolded protein response and fasting-induced steatosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G599–G609 (2016).
Mao, Y. et al. Ghrelin attenuated lipotoxicity via autophagy induction and nuclear factor-κB inhibition. Cell Physiol. Biochem. 37, 563–576 (2015).
Wang, Y., Singh, R., Xiang, Y. & Czaja, M. J. Macroautophagy and chaperone-mediated autophagy are required for hepatocyte resistance to oxidant stress. Hepatology 52, 266–277 (2010).
Papackova, Z., Dankova, H., Palenickova, E., Kazdova, L. & Cahova, M. Effect of short- and long-term high-fat feeding on autophagy flux and lysosomal activity in rat liver. Physiol. Res. 61 (Suppl. 2), S67–S76 (2012).
Ma, D. et al. Autophagy deficiency by hepatic FIP200 deletion uncouples steatosis from liver injury in NAFLD. Mol. Endocrinol. 27, 1643–1654 (2013).
Shibata, M. et al. LC3, a microtubule-associated protein1A/B light chain3, is involved in cytoplasmic lipid droplet formation. Biochem. Biophys. Res. Commun. 393, 274–279 (2010).
Yang, L., Li, P., Fu, S., Calay, E. S. & Hotamisligil, G. S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 (2010).
Baerga, R., Zhang, Y., Chen, P. H., Goldman, S. & Jin, S. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy 5, 1118–1130 (2009).
Singh, R. et al. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest. 119, 3329–3339 (2009).
Zhang, Y. et al. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl Acad. Sci. USA 106, 19860–19865 (2009).
Guo, L. et al. Transactivation of Atg4b by C/EBPbeta promotes autophagy to facilitate adipogenesis. Mol. Cell. Biol. 33, 3180–3190 (2013).
Takagi, A. et al. Mammalian autophagy is essential for hepatic and renal ketogenesis during starvation. Sci. Rep. 6, 18944 (2016).
Martinez-Lopez, N., Athonvarangkul, D., Mishall, P., Sahu, S. & Singh, R. Autophagy proteins regulate ERK phosphorylation. Nat. Commun. 4, 2799 (2013).
Martinez-Lopez, N. et al. Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the brown adipose tissue and liver. Cell Metab. 23, 113–127 (2016).
Peng, Y. et al. ABHD5 interacts with BECN1 to regulate autophagy and tumorigenesis of colon cancer independent of PNPLA2. Autophagy 12, 2167–2182 (2016).
Kaushik, S. & Cuervo, A. M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 17, 759–770 (2015).
Rambold, A. S., Cohen, S. & Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 32, 678–692 (2015).
Fabbrini, E., Sullivan, S. & Klein, S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51, 679–689 (2010).
Samuel, V. T. & Shulman, G. I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 126, 12–22 (2016).
Ginsberg, H. N. & Reyes-Soffer, G. Niacin: a long history, but a questionable future. Curr. Opin. Lipidol 24, 475–479 (2013).
Tunaru, S. et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9, 352–355 (2003).
Lauring, B. et al. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci. Transl. Med. 4, 148ra115 (2012).
Ganji, S. H. et al. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J. Lipid Res. 45, 1835–1845 (2004).
Claus, T. H. et al. Specific inhibition of hormone-sensitive lipase improves lipid profile while reducing plasma glucose. J. Pharmacol. Exp. Ther. 315, 1396–1402 (2005).
Ebdrup, S., Refsgaard, H. H., Fledelius, C. & Jacobsen, P. Synthesis and structure-activity relationship for a novel class of potent and selective carbamate-based inhibitors of hormone selective lipase with acute in vivo antilipolytic effects. J. Med. Chem. 50, 5449–5456 (2007).
Girousse, A. et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 11, e1001485 (2013).
Mayer, N. et al. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat. Chem. Biol. 9, 785–787 (2013).
Schweiger, M. et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat. Commun. 8, 14859 (2017).
Lammers, B. et al. Macrophage adipose triglyceride lipase deficiency attenuates atherosclerotic lesion development in low-density lipoprotein receptor knockout mice. Arterioscler Thromb. Vasc. Biol. 31, 67–73 (2011).
Agustsson, T. et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 67, 5531–5537 (2007).
Das, S. K. et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333, 233–238 (2011).
Ryden, M. et al. Lipolysis—not inflammation, cell death, or lipogenesis—is involved in adipose tissue loss in cancer cachexia. Cancer 113, 1695–1704 (2008).
Rohm, M. et al. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat. Med. 22, 1120–1130 (2016).
Massa, R. et al. Neutral lipid-storage disease with myopathy and extended phenotype with novel PNPLA2 mutation. Muscle Nerve 53, 644–648 (2016).
Natali, A. et al. Metabolic consequences of adipose triglyceride lipase deficiency in humans: an in vivo study in patients with neutral lipid storage disease with myopathy. J. Clin. Endocrinol. Metab. 98, E1540–E1548 (2013).
Dorfman, M. L., Hershko, C., Eisenberg, S. & Sagher, F. Ichthyosiform dermatosis with systemic lipidosis. Arch. Dermatol. 110, 261–266 (1974).
Chanarin, I. et al. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br. Med. J. 1, 553–555 (1975).
Lefevre, C. et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am. J. Hum. Genet. 69, 1002–1012 (2001).
Uchida, Y. et al. Neutral lipid storage leads to acylceramide deficiency, likely contributing to the pathogenesis of Dorfman-Chanarin syndrome. J. Invest. Dermatol. 130, 2497–2499 (2010).
Zhang, J. et al. Comparative gene identification-58 (CGI-58) promotes autophagy as a putative lysophosphatidylglycerol acyltransferase. J. Biol. Chem. 289, 33044–33053 (2014).
Ghosh, A. K., Ramakrishnan, G., Chandramohan, C. & Rajasekharan, R. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J. Biol. Chem. 283, 24525–24533 (2008).
Farhan, S. M. et al. A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. Can. J. Cardiol. 30, 1649–1654 (2014).
Wang, S. P. et al. The catalytic function of hormone-sensitive lipase is essential for fertility in male mice. Endocrinology 155, 3047–3053 (2014).
Abramov, A., Schorr, S. & Wolman, M. Generalized xanthomatosis with calcified adrenals. AMA J. Dis. Child 91, 282–286 (1956).
Sloan, H. R. & Fredrickson, D. S. Enzyme deficiency in cholesteryl ester storage disease. J. Clin. Invest. 51, 1923–1926 (1972).
Burke, J. A. & Schubert, W. K. Deficient activity of acid lipase in cholesterol-ester storage disease. J. Lab Clin. Med. 78, 988–989 (1971).
Patrick, A. D. & Lake, B. D. Deficiency of an acid lipase in Wolman's disease. Nature 222, 1067–1068 (1969).
Bernstein, D. L., Hulkova, H., Bialer, M. G. & Desnick, R. J. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J. Hepatol. 58, 1230–1243 (2013).
Hoffman, E. P., Barr, M. L., Giovanni, M. A. & Murray, M. F. Lysosomal Acid Lipase Deficiency (eds Pagon, R. A. et al.) (University of Washington, 2016).
Burton, B. K. et al. A phase 3 trial of sebelipase alfa in lysosomal acid lipase deficiency. N. Engl. J. Med. 373, 1010–1020 (2015).
Nomura, D. K. et al. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 140, 49–61 (2010).
Zhang, J. et al. Monoacylglycerol lipase: A novel potential therapeutic target and prognostic indicator for hepatocellular carcinoma. Sci. Rep. 6, 35784 (2016).
Nomura, D. K. et al. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem. Biol. 18, 846–856 (2011).
Al-Zoughbi, W. et al. Loss of adipose triglyceride lipase is associated with human cancer and induces mouse pulmonary neoplasia. Oncotarget 7, 33832–33840 (2016).
Zhou, X. et al. Epigenetic downregulation of the ISG15-conjugating enzyme UbcH8 impairs lipolysis and correlates with poor prognosis in nasopharyngeal carcinoma. Oncotarget 6, 41077–41091 (2015).
Wu, J. W. et al. Epistatic interaction between the lipase-encoding genes Pnpla2 and Lipe causes liposarcoma in mice. PLoS Genet. 13, e1006716 (2017).
Miao, H. et al. Macrophage ABHD5 promotes colorectal cancer growth by suppressing spermidine production by SRM. Nat. Commun. 7, 11716 (2016).
Ou, J. et al. Loss of abhd5 promotes colorectal tumor development and progression by inducing aerobic glycolysis and epithelial-mesenchymal transition. Cell Rep. 9, 1798–1811 (2014).
Yan, C., Zhao, T. & Du, H. Lysosomal acid lipase in cancer. Oncoscience 2, 727–728 (2015).
Zhao, T., Du, H., Ding, X., Walls, K. & Yan, C. Activation of mTOR pathway in myeloid-derived suppressor cells stimulates cancer cell proliferation and metastasis in lal−/− mice. Oncogene 34, 1938–1948 (2015).
Du, H., Zhao, T., Ding, X. & Yan, C. Hepatocyte-specific expression of human lysosome acid lipase corrects liver inflammation and tumor metastasis in lal−/− mice. Am. J. Pathol. 185, 2379–2389 (2015).
Zhao, T., Ding, X., Du, H. & Yan, C. Lung epithelial cell-specific expression of human lysosomal acid lipase ameliorates lung inflammation and tumor metastasis in lipa−/− mice. Am. J. Pathol. 186, 2183–2192 (2016).
Tsoli, M. et al. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res. 72, 4372–4382 (2012).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Acknowledgements
Financial support was provided by the European Research Council ERC Grant Agreement 340896, LipoCheX (R.Z.), the DKs Molecular Enzymology (W901) (R.Z.) and Metabolic and Cardiovascular Disease (W1226) (F.M., D.K.), the SFB Lipotox (F30) funded by the Austrian Science Fund (FWF) (R.Z., F.M., D.K.), the Louis-Jeantet Foundation (R.Z.), the Fondation Leducq (grant 12CVD04) (R.Z.) and the BioTechMed-Graz flagship projects EPIAge (F.M.) and Lipid Signalling (D.K.). F.M. acknowledges additional support from NAWI Graz; BioTechMed-Graz (“EPIAge”); FWF grants P29262, P29203 and P27893; and the BMWFW and University of Graz grants “Unkonventionelle Forschung” and “Flysleep”.
Author information
Authors and Affiliations
Contributions
Researching the literature for the article: R.Z., F.M. and D.K.; substantial contributions to discussion of the content: R.Z., F.M. and D.K.; writing: R.Z. and D.K.; review and/or editing of the manuscript before submission: R.Z., F.M. and D.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Fatty acids
-
Monocarboxylic acids with long saturated or unsaturated aliphatic carbon chains. They are either unesterified, in free form (free fatty acids) or esterified to various alcohols.
- Acid–base homeostasis
-
Maintenance of a constant pH in extracellular body fluids by balanced concentrations of acids and bases.
- White adipose tissue
-
(WAT). A loose connective tissue that predominantly consists of adipocytes and that stores excess nutrients as triacylglycerols.
- Very-low-density lipoproteins
-
(VLDL). Liver-derived plasma lipoproteins of very low density that transport lipids (predominantly triacylglycerols) from the liver to non-hepatic tissues.
- Chylomicrons
-
Intestine-derived plasma lipoproteins that transport dietary lipids (predominantly triacylglycerols) from the digestive tract (intestine) to the liver and other tissues.
- Lipases
-
Enzymes that hydrolyse fatty acid–glycerol esters.
- Patatin-like phospholipase domain-containing protein 2
-
(PNPLA2). A protein with a patatin domain that hydrolyses neutral lipids, phospholipids and retinyl esters.
- Patatin domain
-
A protein domain of approximately 180 amino acids in length that was originally discovered in the potato tuber storage protein patatin.
- Brown adipose tissue
-
Adipose tissue involved in thermoregulation, uncoupling mitochondrial electron transport from ATP synthesis and thereby generating heat during chronic cold exposure.
- sn
-
A notation that stands for 'stereospecific numbering' and describes the stereochemical configuration of chiral glycerol derivatives.
- Endocannabinoid signalling
-
Signalling that comprises cannabinoid receptors and their endogenous ligands, which regulate numerous physiological processes, including energy homeostasis, pain perception, inflammation and tumorigenesis.
- Catecholamines
-
Tyrosine derivatives of catechol, including adrenaline, noradrenaline and dopamine, that act as neuromodulators and hormones.
- Natriuretic peptides
-
Peptides that control the homeostasis of water, sodium and potassium in the body. In adipocytes, these peptides also regulate lipolysis.
- Scavenger receptors
-
Lipoprotein receptors that remove modified lipoproteins (for example, acetylated or oxidized low-density lipoproteins) and other negatively charged macromolecules from the blood.
- Kupffer cells
-
Specialized macrophages in the liver.
- Foam cell
-
A lipid-filled macrophage that is present in atherosclerotic lesions and plaques.
- Steatosis
-
A process describing the abnormal retention of neutral lipids (triacylglycerols and cholesteryl esters) within cells and tissues.
- Fatty liver disease
-
A reversible condition characterized by the excessive accumulation of neutral lipids in the liver. This condition can be subdivided into alcoholic fatty liver disease and non-alcoholic fatty liver disease.
- Cachexia
-
A wasting syndrome characterized by an unintentional and nutritionally irreversible loss of body mass (muscle and fat mass). Various chronic diseases can lead to cachexia, but it is most common in cancer.
Rights and permissions
About this article
Cite this article
Zechner, R., Madeo, F. & Kratky, D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol 18, 671–684 (2017). https://doi.org/10.1038/nrm.2017.76
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2017.76
This article is cited by
-
DDHD2, whose mutations cause spastic paraplegia type 54, enhances lipophagy via engaging ATG8 family proteins
Cell Death & Differentiation (2024)
-
ATG14 targets lipid droplets and acts as an autophagic receptor for syntaxin18-regulated lipid droplet turnover
Nature Communications (2024)
-
Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology
Nature Reviews Molecular Cell Biology (2024)
-
Glucose controls lipolysis through Golgi PtdIns4P-mediated regulation of ATGL
Nature Cell Biology (2024)
-
Lipid droplets and cellular lipid flux
Nature Cell Biology (2024)