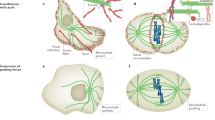
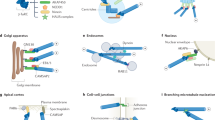
Key Points
-
Assembly of the microtubule nucleus is an energetically unfavourable process.
-
Microtubule nucleation is stimulated and can be controlled by the following two principal activities: by providing a stable template (such as the γ-tubulin ring complex (γTuRC) or a severed microtubule) or by the action of microtubule-associated proteins (MAPs) that stabilize the nascent microtubule 'nucleus'.
-
MAPs with the potential to promote microtubule nucleation include the microtubule polymerases of the XMAP215 family and suppressors of microtubule dynamics.
-
These stabilizing MAPs are also likely to provide an additional layer of control over microtubule nucleation by counteracting microtubule destabilizers that have been shown to inhibit nucleation.
-
The different activities most likely act synergistically in the cell environment to facilitate efficient microtubule nucleation.
Abstract
Microtubules are cytoskeletal filaments central to a wide range of essential cellular functions in eukaryotic cells. Consequently, cells need to exert tight control over when, where and how many microtubules are being made. Whereas the regulation of microtubule dynamics is well studied, the molecular mechanisms of microtubule nucleation are still poorly understood. Next to the established master template of nucleation, the γ-tubulin ring complex, other microtubule-associated proteins that affect microtubule dynamic properties have recently been found to contribute to nucleation. It has begun to emerge that the nucleation efficiency is controlled not only by template activity but also by, either additionally or alternatively, the stabilization of the nascent microtubule 'nucleus'. This suggests a simple conceptual framework for the mechanisms regulating microtubule nucleation in cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Keating, T. J. & Borisy, G. G. Centrosomal and non-centrosomal microtubules. Biol. Cell 91, 321–329 (1999).
Sanchez, A. D. & Feldman, J. L. Microtubule-organizing centers: from the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. 44, 93–101 (2017).
Kirschner, M. & Mitchison, T. Beyond self-assembly: from microtubules to morphogenesis. Cell 45, 329–342 (1986).
Lindeboom, J. J. et al. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342, 1245533 (2013).
Helmke, K. J., Heald, R. & Wilbur, J. D. Interplay between spindle architecture and function. Int. Rev. Cell. Mol. Biol. 306, 83–125 (2013).
Gardner, M. K., Zanic, M. & Howard, J. Microtubule catastrophe and rescue. Curr. Opin. Cell Biol. 25, 14–22 (2013).
Nogales, E. & Zhang, R. Visualizing microtubule structural transitions and interactions with associated proteins. Curr. Opin. Struct. Biol. 37, 90–96 (2016).
Kollman, J. M., Merdes, A., Mourey, L. & Agard, D. A. Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Biol. 12, 709–721 (2011).
Teixido-Travesa, N., Roig, J. & Luders, J. The where, when and how of microtubule nucleation — one ring to rule them all. J. Cell Sci. 125, 4445–4456 (2012).
Lin, T. C., Neuner, A. & Schiebel, E. Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence. Trends Cell Biol. 25, 296–307 (2015).
Voter, W. A. & Erickson, H. P. The kinetics of microtubule assembly. Evidence for a two-stage nucleation mechanism. J. Biol. Chem. 259, 10430–10438 (1984).
Fygenson, D. K., Flyvbjerg, H., Sneppen, K., Libchaber, A. & Leibler, S. Spontaneous nucleation of microtubules. Phys. Rev. E 51, 5058–5063 (1995).
Wieczorek, M., Bechstedt, S., Chaaban, S. & Brouhard, G. J. Microtubule-associated proteins control the kinetics of microtubule nucleation. Nat. Cell Biol. 17, 907–916 (2015).
McIntosh, J. R., Morphew, M. K., Grissom, P. M., Gilbert, S. P. & Hoenger, A. Lattice structure of cytoplasmic microtubules in a cultured mammalian cell. J. Mol. Biol. 394, 177–182 (2009).
Ray, S., Meyhofer, E., Milligan, R. A. & Howard, J. Kinesin follows the microtubule's protofilament axis. J. Cell Biol. 121, 1083–1093 (1993).
Erickson, H. P. & Pantaloni, D. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophys. J. 34, 293–309 (1981).
Alushin, G. M. et al. High-resolution microtubule structures reveal the structural transitions in αβ-tubulin upon GTP hydrolysis. Cell 157, 1117–1129 (2014).
Hyman, A. A., Salser, S., Drechsel, D. N., Unwin, N. & Mitchison, T. J. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue. GMPCPP. Mol. Biol. Cell 3, 1155–1167 (1992).
Hamel, E., del Campo, A. A., Lowe, M. C. & Lin, C. M. Interactions of taxol, microtubule-associated proteins, and guanine nucleotides in tubulin polymerization. J. Biol. Chem. 256, 11887–11894 (1981).
Arnal, I. & Wade, R. H. How does taxol stabilize microtubules? Curr. Biol. 5, 900–908 (1995).
Lee, J. C., Hirsh, J. & Timasheff, S. N. In vitro formation of filaments from calf brain microtubule protein. Arch. Biochem. Biophys. 168, 726–729 (1975).
Kellogg, E. H. et al. Insights into the distinct mechanisms of action of taxane and non-taxane microtubule stabilizers from cryo-EM structures. J. Mol. Biol. 429, 633–646 (2017).
Elie-Caille, C. et al. Straight GDP-tubulin protofilaments form in the presence of taxol. Curr. Biol. 17, 1765–1770 (2007).
Yajima, H. et al. Conformational changes in tubulin in GMPCPP and GDP-taxol microtubules observed by cryoelectron microscopy. J. Cell Biol. 198, 315–322 (2012).
Lee, J. C. & Timasheff, S. N. In vitro reconstitution of calf brain microtubules: effects of solution variables. Biochemistry 16, 1754–1764 (1977).
Flyvbjerg, H. & Jobs, E. Microtubule dynamics. II. Kinetics of self-assembly. Phys. Rev. E 56, 7083–7099 (1997).
Cooper, J. A. et al. Kinetic evidence for a monomer activation step in actin polymerization. Biochemistry 22, 2193–2202 (1983).
Tobacman, L. S. & Korn, E. D. The kinetics of actin nucleation and polymerization. J. Biol. Chem. 258, 3207–3214 (1983).
Mozziconacci, J., Sandblad, L., Wachsmuth, M., Brunner, D. & Karsenti, E. Tubulin dimers oligomerize before their incorporation into microtubules. PLoS ONE 3, e3821 (2008).
Wang, H. W., Long, S., Finley, K. R. & Nogales, E. Assembly of GMPCPP-bound tubulin into helical ribbons and tubes and effect of colchicine. Cell Cycle 4, 1157–1160 (2005).
Portran, D., Schaedel, L., Xu, Z., Thery, M. & Nachury, M. V. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 19, 391–398 (2017).
Mandelkow, E. M., Mandelkow, E. & Milligan, R. A. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J. Cell Biol. 114, 977–991 (1991).
Sui, H. & Downing, K. H. Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure 18, 1022–1031 (2010).
Gard, D. L. & Kirschner, M. W. Microtubule assembly in cytoplasmic extracts of Xenopus oocytes and eggs. J. Cell Biol. 105, 2191–2201 (1987).
Belmont, L. D. & Mitchison, T. J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell 84, 623–631 (1996).
Stearns, T. & Kirschner, M. In vitro reconstitution of centrosome assembly and function: the central role of γ-tubulin. Cell 76, 623–637 (1994).
Zheng, Y., Wong, M. L., Alberts, B. & Mitchison, T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature 378, 578–583 (1995).
Moritz, M., Braunfeld, M. B., Guenebaut, V., Heuser, J. & Agard, D. A. Structure of the γ-tubulin ring complex: a template for microtubule nucleation. Nat. Cell Biol. 2, 365–370 (2000).
Wiese, C. & Zheng, Y. A new function for the γ-tubulin ring complex as a microtubule minus-end cap. Nat. Cell Biol. 2, 358–364 (2000).
Aldaz, H., Rice, L. M., Stearns, T. & Agard, D. A. Insights into microtubule nucleation from the crystal structure of human γ-tubulin. Nature 435, 523–527 (2005).
Kollman, J. M., Polka, J. K., Zelter, A., Davis, T. N. & Agard, D. A. Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry. Nature 466, 879–882 (2010).
Oegema, K. et al. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144, 721–733 (1999).
Choi, Y. K., Liu, P., Sze, S. K., Dai, C. & Qi, R. Z. CDK5RAP2 stimulates microtubule nucleation by the γ-tubulin ring complex. J. Cell Biol. 191, 1089–1095 (2010).
Lin, T. C. et al. Cell-cycle dependent phosphorylation of yeast pericentrin regulates γ-TuSC-mediated microtubule nucleation. eLife 3, e02208 (2014).
Kollman, J. M. et al. Ring closure activates yeast γTuRC for species-specific microtubule nucleation. Nat. Struct. Mol. Biol. 22, 132–137 (2015).
Flyvbjerg, H., Jobs, E. & Leibler, S. Kinetics of self-assembling microtubules: an “inverse problem” in biochemistry. Proc. Natl Acad. Sci. USA 93, 5975–5979 (1996).
McNally, F. J. & Vale, R. D. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell 75, 419–429 (1993).
Srayko, M., O'Toole, E., T., Hyman, A. A. & Muller-Reichert, T. Katanin disrupts the microtubule lattice and increases polymer number in C. elegans meiosis. Curr. Biol. 16, 1944–1949 (2006).
Baas, P. W. & Ahmad, F. J. The plus ends of stable microtubules are the exclusive nucleating structures for microtubules in the axon. J. Cell Biol. 116, 1231–1241 (1992).
Davis, L. J., Odde, D. J., Block, S. M. & Gross, S. P. The importance of lattice defects in katanin-mediated microtubule severing in vitro. Biophys. J. 82, 2916–2927 (2002).
White, S. R., Evans, K. J., Lary, J., Cole, J. L. & Lauring, B. Recognition of C-terminal amino acids in tubulin by pore loops in Spastin is important for microtubule severing. J. Cell Biol. 176, 995–1005 (2007).
Roll-Mecak, A. & Vale, R. D. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature 451, 363–367 (2008).
Nakamura, M. Microtubule nucleating and severing enzymes for modifying microtubule array organization and cell morphogenesis in response to environmental cues. New Phytol. 205, 1022–1027 (2015).
Sharp, D. J. & Ross, J. L. Microtubule-severing enzymes at the cutting edge. J. Cell Sci. 125, 2561–2569 (2012).
O'Toole, E., Greenan, G., Lange, K. I., Srayko, M. & Muller-Reichert, T. The role of γ-tubulin in centrosomal microtubule organization. PLoS ONE 7, e29795 (2012).
Strome, S. et al. Spindle dynamics and the role of γ-tubulin in early Caenorhabditis elegans embryos. Mol. Biol. Cell 12, 1751–1764 (2001).
Sampaio, P., Rebollo, E., Varmark, H., Sunkel, C. E. & Gonzalez, C. Organized microtubule arrays in γ-tubulin-depleted Drosophila spermatocytes. Curr. Biol. 11, 1788–1793 (2001).
Hannak, E. et al. The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is γ-tubulin dependent. J. Cell Biol. 157, 591–602 (2002).
Yau, K. W. et al. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron 82, 1058–1073 (2014).
Nakaoka, Y., Kimura, A., Tani, T. & Goshima, G. Cytoplasmic nucleation and atypical branching nucleation generate endoplasmic microtubules in Physcomitrella patens. Plant Cell 27, 228–242 (2015).
Rogers, G. C., Rusan, N. M., Peifer, M. & Rogers, S. L. A multicomponent assembly pathway contributes to the formation of acentrosomal microtubule arrays in interphase Drosophila cells. Mol. Biol. Cell 19, 3163–3178 (2008).
Kitamura, E. et al. Kinetochores generate microtubules with distal plus ends: their roles and limited lifetime in mitosis. Dev. Cell 18, 248–259 (2010).
Nashchekin, D., Fernandes, A. R. & St Johnston, D. Patronin/Shot cortical foci assemble the noncentrosomal microtubule array that specifies the Drosophila anterior-posterior axis. Dev. Cell 38, 61–72 (2016).
Gard, D. L. & Kirschner, M. W. A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J. Cell Biol. 105, 2203–2215 (1987).
Brouhard, G. J. et al. XMAP215 is a processive microtubule polymerase. Cell 132, 79–88 (2008).
Podolski, M., Mahamdeh, M. & Howard, J. Stu2, the budding yeast XMAP215/Dis1 homolog, promotes assembly of yeast microtubules by increasing growth rate and decreasing catastrophe frequency. J. Biol. Chem. 289, 28087–28093 (2014).
Li, W. et al. Reconstitution of dynamic microtubules with Drosophila XMAP215, EB1, and Sentin. J. Cell Biol. 199, 849–862 (2012).
Roostalu, J., Cade, N. I. & Surrey, T. Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nat. Cell Biol. 17, 1422–1434 (2015).
Al-Bassam, J. et al. Fission yeast Alp14 is a dose-dependent plus end-tracking microtubule polymerase. Mol. Biol. Cell 23, 2878–2890 (2012).
Gard, D. L., Becker, B. E. & Romney, S. J. MAPping the eukaryotic tree of life: structure, function, and evolution of the MAP215/Dis1 family of microtubule-associated proteins. Int. Rev. Cytol. 239, 179–272 (2004).
Al-Bassam, J., Larsen, N. A., Hyman, A. A. & Harrison, S. C. Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure 15, 355–362 (2007).
Widlund, P. O. et al. XMAP215 polymerase activity is built by combining multiple tubulin-binding TOG domains and a basic lattice-binding region. Proc. Natl Acad. Sci. USA 108, 2741–2746 (2011).
Ayaz, P. et al. A tethered delivery mechanism explains the catalytic action of a microtubule polymerase. eLife 3, e03069 (2014).
Ayaz, P., Ye, X., Huddleston, P., Brautigam, C. A. & Rice, L. M. A. TOG: αβ-tubulin complex structure reveals conformation-based mechanisms for a microtubule polymerase. Science 337, 857–860 (2012).
Al-Bassam, J. & Chang, F. Regulation of microtubule dynamics by TOG-domain proteins XMAP215/Dis1 and CLASP. Trends Cell Biol. 21, 604–614 (2011).
Kosco, K. A. et al. Control of microtubule dynamics by Stu2p is essential for spindle orientation and metaphase chromosome alignment in yeast. Mol. Biol. Cell 12, 2870–2880 (2001).
Graf, R., Euteneuer, U., Ho, T. H. & Rehberg, M. Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number. Mol. Biol. Cell 14, 4067–4074 (2003).
Kawamura, E. et al. MICROTUBULE ORGANIZATION 1 regulates structure and function of microtubule arrays during mitosis and cytokinesis in the Arabidopsis root. Plant Physiol. 140, 102–114 (2006).
Groen, A. C., Maresca, T. J., Gatlin, J. C., Salmon, E. D. & Mitchison, T. J. Functional overlap of microtubule assembly factors in chromatin-promoted spindle assembly. Mol. Biol. Cell 20, 2766–2773 (2009).
Tournebize, R. et al. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat. Cell Biol. 2, 13–19 (2000).
Kronja, I., Kruljac-Letunic, A., Caudron-Herger, M., Bieling, P. & Karsenti, E. XMAP215-EB1 interaction is required for proper spindle assembly and chromosome segregation in Xenopus egg extract. Mol. Biol. Cell 20, 2684–2696 (2009).
Popov, A. V., Severin, F. & Karsenti, E. XMAP215 is required for the microtubule-nucleating activity of centrosomes. Curr. Biol. 12, 1326–1330 (2002).
Ghosh, S., Hentrich, C. & Surrey, T. Micropattern-controlled local microtubule nucleation, transport, and mesoscale organization. ACS Chem. Biol. 8, 673–678 (2013).
Wittmann, T., Wilm, M., Karsenti, E. & Vernos, I. TPX2, a novel xenopus MAP involved in spindle pole organization. J. Cell Biol. 149, 1405–1418 (2000).
Gruss, O. J. et al. Ran induces spindle assembly by reversing the inhibitory effect of importin α on TPX2 activity. Cell 104, 83–93 (2001).
Petry, S., Groen, A. C., Ishihara, K., Mitchison, T. J. & Vale, R. D. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768–777 (2013).
Brunet, S. et al. Meiotic regulation of TPX2 protein levels governs cell cycle progression in mouse oocytes. PloS ONE 3, e3338 (2008).
Gruss, O. J. et al. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4, 871–879 (2002).
Bayliss, R., Sardon, T., Vernos, I. & Conti, E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 12, 851–862 (2003).
Schatz, C. A. et al. Importin α-regulated nucleation of microtubules by TPX2. EMBO J. 22, 2060–2070 (2003).
Brunet, S. et al. Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts. Mol. Biol. Cell 15, 5318–5328 (2004).
Reid, T. A. et al. Suppression of microtubule assembly kinetics by the mitotic protein TPX2. J. Cell Sci. 129, 1319–1328 (2016).
Al-Bassam, J. et al. CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Dev. Cell 19, 245–258 (2010).
Moriwaki, T. & Goshima, G. Five factors can reconstitute all three phases of microtubule polymerization dynamics. J. Cell Biol. 215, 357–368 (2016).
Hannak, E. & Heald, R. Xorbit/CLASP links dynamic microtubules to chromosomes in the Xenopus meiotic spindle. J. Cell Biol. 172, 19–25 (2006).
Efimov, A. et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12, 917–930 (2007).
Wu, J. et al. Molecular pathway of microtubule organization at the Golgi apparatus. Dev. Cell 39, 44–60 (2016).
Jiang, K. et al. Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev. Cell 28, 295–309 (2014).
Hendershott, M. C. & Vale, R. D. Regulation of microtubule minus-end dynamics by CAMSAPs and Patronin. Proc. Natl Acad. Sci. USA 111, 5860–5865 (2014).
Goodwin, S. S. & Vale, R. D. Patronin regulates the microtubule network by protecting microtubule minus ends. Cell 143, 263–274 (2010).
Wang, S. et al. NOCA-1 functions with gamma- tubulin and in parallel to Patronin to assemble non-centrosomal microtubule arrays in C. elegans. eLife 4, e08649 (2015).
Tanaka, N., Meng, W., Nagae, S. & Takeichi, M. Nezha/CAMSAP3 and CAMSAP2 cooperate in epithelial-specific organization of noncentrosomal microtubules. Proc. Natl Acad. Sci. USA 109, 20029–20034 (2012).
Lazarus, J. E., Moughamian, A. J., Tokito, M. K. & Holzbaur, E. L. Dynactin subunit p150(Glued) is a neuron-specific anti-catastrophe factor. PLoS Biol. 11, e1001611 (2013).
Prosser, S. L. & Pelletier, L. Mitotic spindle assembly in animal cells: a fine balancing act. Nat. Rev. Mol. Cell Biol. 18, 187–201 (2017).
Ho, C. M. et al. Augmin plays a critical role in organizing the spindle and phragmoplast microtubule arrays in Arabidopsis. Plant Cell 23, 2606–2618 (2011).
Liu, T. et al. Augmin triggers microtubule-dependent microtubule nucleation in interphase plant cells. Curr. Biol. 24, 2708–2713 (2014).
Nakaoka, Y. et al. An inducible RNA interference system in Physcomitrella patens reveals a dominant role of augmin in phragmoplast microtubule generation. Plant Cell 24, 1478–1493 (2012).
Hsia, K. C. et al. Reconstitution of the augmin complex provides insights into its architecture and function. Nat. Cell Biol. 16, 852–863 (2014).
Goshima, G., Mayer, M., Zhang, N., Stuurman, N. & Vale, R. D. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 181, 421–429 (2008).
Goshima, G. et al. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316, 417–421 (2007).
Chretien, D., Fuller, S. D. & Karsenti, E. Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. J. Cell Biol. 129, 1311–1328 (1995).
Brouhard, G. J. & Rice, L. M. The contribution of αβ-tubulin curvature to microtubule dynamics. J. Cell Biol. 207, 323–334 (2014).
Maurer, S. P., Bieling, P., Cope, J., Hoenger, A. & Surrey, T. GTPγS microtubules mimic the growing microtubule end structure recognized by end-binding proteins (EBs). Proc. Natl Acad. Sci. USA 108, 3988–3993 (2011).
Seetapun, D., Castle, B. T., McIntyre, A. J., Tran, P. T. & Odde, D. J. Estimating the microtubule GTP cap size in vivo. Curr. Biol. 22, 1681–1687 (2012).
Duellberg, C., Cade, N. I., Holmes, D. & Surrey, T. The size of the EB cap determines instantaneous microtubule stability. eLife 5, e13470 (2016).
Gardner, M. K. et al. Rapid microtubule self-assembly kinetics. Cell 146, 582–592 (2011).
VanBuren, V., Odde, D. J. & Cassimeris, L. Estimates of lateral and longitudinal bond energies within the microtubule lattice. Proc. Natl Acad. Sci. USA 99, 6035–6040 (2002).
Mitchison, T. & Kirschner, M. Dynamic instability of microtubule growth. Nature 312, 237–242 (1984).
Nogales, E. An electron microscopy journey in the study of microtubule structure and dynamics. Protein Sci. 24, 1912–1919 (2015).
Gekko, K. & Timasheff, S. N. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry 20, 4667–4676 (1981).
Nogales, E., Wolf, S. G., Khan, I. A., Luduena, R. F. & Downing, K. H. Structure of tubulin at 6.5 A and location of the taxol-binding site. Nature 375, 424–427 (1995).
Acknowledgements
This work was supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001163), the UK Medical Research Council (FC001163), and the Wellcome Trust (FC001163). J.R acknowledges funding from a Sir Henry Wellcome Postdoctoral Fellowship (100145/Z/12/Z), and T.S., from the European Research Council (Advanced Grant, project 323042).
Author information
Authors and Affiliations
Contributions
Both T.S. and J.R. discussed the content of the manuscript and contributed substantially to the writing and review/editing of the manuscript before its submission. J.R. assembled the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Plus end
-
Fast growing end of the polar microtubule filament, with exposed β-tubulin at its terminus (Box 1).
- Minus end
-
Slowly growing end of the polar microtubule filament, with exposed α-tubulin at its terminus (Box 1).
- Cortical microtubules
-
Ordered acentrosomal array of microtubules at the plant cell cortex that is important for cell morphogenesis.
- Stathmin/Op18
-
Small, 18 kDa, disordered protein that acts as a tubulin-sequestering factor by binding simultaneously to two tubulin dimers, thereby lowering the polymerization-competent tubulin concentration in the living cell.
- Centrosomes
-
Non-membranous organelles that act as the main microtubule organizing and nucleating centres in somatic animal cells.
- Axonemes
-
Microtubule-based core structural components of cilia consisting of either 9 microtubules organized in a circle (primary cilia) or 9 microtubule doublets organized in a circle with an additional microtubule pair in the middle (motile cilia).
- TOG domains
-
Paddle-shaped tubulin-binding domains composed of six HEAT repeat motifs found in XMAP215, CLASP and crescerin family proteins.
- Kinetochore
-
Large multi-protein complex usually assembled on centromeric DNA that serves as a site of interaction between chromosomes and microtubules.
- Aurora A kinase
-
Serine/threonine kinase with numerous functions in mitosis and meiosis, including centrosome maturation and spindle assembly.
Rights and permissions
About this article
Cite this article
Roostalu, J., Surrey, T. Microtubule nucleation: beyond the template. Nat Rev Mol Cell Biol 18, 702–710 (2017). https://doi.org/10.1038/nrm.2017.75
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2017.75
This article is cited by
-
CAMSAPs and nucleation-promoting factors control microtubule release from γ-TuRC
Nature Cell Biology (2024)
-
Structure of the native γ-tubulin ring complex capping spindle microtubules
Nature Structural & Molecular Biology (2024)
-
Microtubules provide force to promote membrane uncoating in vacuolar escape for a cyto-invasive bacterial pathogen
Nature Communications (2024)
-
Structural insights into the mechanism of GTP initiation of microtubule assembly
Nature Communications (2023)
-
The phosphorylation of PHF5A by TrkA-ERK1/2-ABL1 cascade regulates centrosome separation
Cell Death & Disease (2023)