Key Points
-
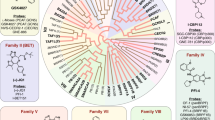
Bromodomains (BRDs) are evolutionarily conserved protein–protein interaction modules. Structure-based alignments have clustered human BRDs into eight distinct families.
-
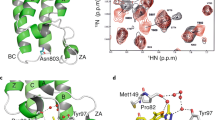
BRD modules share a conserved bundle of 4 α-helices (αZ, αA, αB and αC) that are linked to each other by loop segments of variable length (ZA and BC loops).
-
BRDs primarily recognize acetylated Lys residues on histones. Several BRDs were found to recognize acetylated non-histone proteins.
-
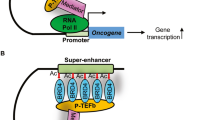
BRD-containing proteins regulate gene expression, alone or as part of larger protein complexes, through chromatin remodelling, histone modification, histone recognition and transcriptional machinery regulation.
-
BRD-containing proteins are frequently deregulated in cancer, and mutations in the BRDs themselves are frequently identified in a variety of cancers. BRD-containing proteins also form part of oncogenic fusion proteins that result from chromosomal rearrangements.
-
The development of BRD inhibitors as anticancer agents is now an intense area of research. Small-molecule inhibitors that target the bromodomain and extraterminal domain (BET) family of BRDs are now being tested in clinical trials for the treatment of various types of cancers.
Abstract
Bromodomains (BRDs) are evolutionarily conserved protein–protein interaction modules that are found in a wide range of proteins with diverse catalytic and scaffolding functions and are present in most tissues. BRDs selectively recognize and bind to acetylated Lys residues — particularly in histones — and thereby have important roles in the regulation of gene expression. BRD-containing proteins are frequently dysregulated in cancer, they participate in gene fusions that generate diverse, frequently oncogenic proteins, and many cancer-causing mutations have been mapped to the BRDs themselves. Importantly, BRDs can be targeted by small-molecule inhibitors, which has stimulated many translational research projects that seek to attenuate the aberrant functions of BRD-containing proteins in disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kim, S. C. et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23, 607–618 (2006). The first proteomic study to report the widespread existence of acetylation in human (HeLa) and mouse cells.
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009). A large-scale, high-resolution proteomic screen that identified over 3,500 acetylated Lys sites in human cells.
Choudhary, C., Weinert, B. T., Nishida, Y., Verdin, E. & Mann, M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550 (2014).
Verdin, E. & Ott, M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 16, 258–264 (2015).
Bannister, A. J. & Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 21, 381–395 (2011).
Glozak, M. A. & Seto, E. Histone deacetylases and cancer. Oncogene 26, 5420–5432 (2007).
Zhao, D., Li, F. L., Cheng, Z. L. & Lei, Q. Y. Impact of acetylation on tumor metabolism. Mol. Cell. Oncol. 1, e963452 (2014).
Falkenberg, K. J. & Johnstone, R. W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 13, 673–691 (2014).
Haynes, S. R. et al. The bromodomain: a conserved sequence found in human, Drosophila and yeast proteins. Nucleic Acids Res. 20, 2603 (1992). The first report of the BRD motif, which speculates that it constitutes a protein–protein interaction domain.
Li, Y. et al. AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell 159, 558–571 (2014).
Li, Y. et al. Molecular coupling of histone crotonylation and active transcription by AF9 YEATS domain. Mol. Cell 62, 181–193 (2016).
Andrews, F. H. et al. The Taf14 YEATS domain is a reader of histone crotonylation. Nat. Chem. Biol. 12, 396–398 (2016).
Kim, M. S. et al. A draft map of the human proteome. Nature 509, 575–581 (2014).
Wilhelm, M. et al. Mass-spectrometry-based draft of the human proteome. Nature 509, 582–584 (2014).
Muller, S., Filippakopoulos, P. & Knapp, S. Bromodomains as therapeutic targets. Expert Rev. Mol. Med. 13, e29 (2011).
Belkina, A. C. & Denis, G. V. BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer 12, 465–477 (2012).
Shi, J. & Vakoc, C. R. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol. Cell 54, 728–736 (2014).
Wang, C. Y. & Filippakopoulos, P. Beating the odds: BETs in disease. Trends Biochem. Sci. 40, 468–479 (2015).
Filippakopoulos, P. & Knapp, S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat. Rev. Drug Discov. 13, 337–356 (2014).
Basheer, F. & Huntly, B. J. BET bromodomain inhibitors in leukemia. Exp. Hematol. 43, 718–731 (2015).
Theodoulou, N. H., Tomkinson, N. C., Prinjha, R. K. & Humphreys, P. G. Progress in the development of non-BET bromodomain chemical probes. ChemMedChem 11, 477–487 (2016).
Filippakopoulos, P. et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 149, 214–231 (2012). This study reported the first large-scale structural analysis of the human BRD family.
Alsarraj, J. et al. BRD4 short isoform interacts with RRP1B, SIPA1 and components of the LINC complex at the inner face of the nuclear membrane. PLoS ONE 8, e80746 (2013).
Dhalluin, C. et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature 399, 491–496 (1999). The first report of the solution structure of a BRD (from PCAF), which established that it interacts directly with acetylated Lys residues.
Jacobson, R. H., Ladurner, A. G., King, D. S. & Tjian, R. Structure and function of a human TAFII250 double bromodomain module. Science 288, 1422–1425 (2000). The first crystal structure of a tandem BRD module from TAF1, which established the rationale for the simultaneous engagement of multiple acetylated histone peptides.
Owen, D. J. et al. The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase Gcn5p. EMBO J. 19, 6141–6149 (2000). The first high-resolution crystal structure of the yeast Gcn5 BRD bound to an acetylated H4 peptide.
Mujtaba, S. et al. Structural basis of lysine-acetylated HIV-1 Tat recognition by PCAF bromodomain. Mol. Cell 9, 575–586 (2002).
Mujtaba, S. et al. Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol. Cell 13, 251–263 (2004).
Gamsjaeger, R. et al. Structural basis and specificity of acetylated transcription factor GATA1 recognition by BET family bromodomain protein Brd3. Mol. Cell. Biol. 31, 2632–2640 (2011). This study demonstrated that BRD3 recognizes acetylated Lys residues in the transcription factor GATA1, which participates in the regulation of haematopoietic lineages.
Schroder, S. et al. Two-pronged binding with bromodomain-containing protein 4 liberates positive transcription elongation factor b from inactive ribonucleoprotein complexes. J. Biol. Chem. 287, 1090–1099 (2012).
Shi, J. et al. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell 25, 210–225 (2014). This study demonstrated that BRD4 recognizes acetylated Lys residues on the transcription factor TWIST, which affects TWIST-controlled gene expression programmes.
Zou, Z. et al. Brd4 maintains constitutively active NF-κB in cancer cells by binding to acetylated RelA. Oncogene 33, 2395–2404 (2014).
Tsai, W. W. et al. TRIM24 links a non-canonical histone signature to breast cancer. Nature 468, 927–932 (2010). This study demonstrated that the PHD–BRD cassette of TRIM24 combinatorially recognizes H3K23ac and unmodified H3K4.
Li, H. et al. Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature 442, 91–95 (2006).
Ruthenburg, A. J. et al. Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell 145, 692–706 (2011). This study demonstrated that BPTF multivalently recognizes Kac histone marks on the tails of different histones within the same nucleosome.
Xi, Q. et al. A poised chromatin platform for TGF-β access to master regulators. Cell 147, 1511–1524 (2011).
Moriniere, J. et al. Cooperative binding of two acetylation marks on a histone tail by a single bromodomain. Nature 461, 664–668 (2009). The first report to show that a single BRD can recognize two Kac histone marks simultaneously.
Chen, Y. et al. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell. Proteomics 6, 812–819 (2007).
Tan, M. et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146, 1016–1028 (2011).
Sabari, B. R. et al. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell 58, 203–215 (2015).
Goudarzi, A. et al. Dynamic competing histone H4 K5K8 acetylation and butyrylation are hallmarks of highly active gene promoters. Mol. Cell 62, 169–180 (2016). This study demonstrated that histone butyrylation regulates the binding of BRDT to histones.
Rousseaux, S. & Khochbin, S. Histone acylation beyond acetylation: terra incognita in chromatin biology. Cell J. 17, 1–6 (2015).
Flynn, E. M. et al. A subset of human bromodomains recognizes butyryllysine and crotonyllysine histone peptide modifications. Structure 23, 1801–1814 (2015).
Singhal, N. et al. Chromatin-remodeling components of the BAF complex facilitate reprogramming. Cell 141, 943–955 (2010).
Singh, M., Popowicz, G. M., Krajewski, M. & Holak, T. A. Structural ramification for acetyl-lysine recognition by the bromodomain of human BRG1 protein, a central ATPase of the SWI/SNF remodeling complex. Chembiochem 8, 1308–1316 (2007).
Reyes, J. C. et al. Altered control of cellular proliferation in the absence of mammalian Brahma (SNF2α). EMBO J. 17, 6979–6991 (1998).
Bultman, S. et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol. Cell 6, 1287–1295 (2000).
Huang, X., Gao, X., Diaz-Trelles, R., Ruiz-Lozano, P. & Wang, Z. Coronary development is regulated by ATP-dependent SWI/SNF chromatin remodeling component BAF180. Dev. Biol. 319, 258–266 (2008).
Burrows, A. E., Smogorzewska, A. & Elledge, S. J. Polybromo-associated BRG1-associated factor components BRD7 and BAF180 are critical regulators of p53 required for induction of replicative senescence. Proc. Natl Acad. Sci. USA 107, 14280–14285 (2010).
Brownlee, P. M., Chambers, A. L., Cloney, R., Bianchi, A. & Downs, J. A. BAF180 promotes cohesion and prevents genome instability and aneuploidy. Cell Rep. 6, 973–981 (2014).
Kaeser, M. D. et al. BRD7, a novel PBAF-specific SWI/SNF subunit, is required for target gene activation and repression in embryonic stem cells. J. Biol. Chem. 283, 32254–32263 (2008).
Chiu, Y. H., Lee, J. Y. & Cantley, L. C. BRD7, a tumor suppressor, interacts with p85α and regulates PI3K activity. Mol. Cell 54, 193–202 (2014). This study showed that BRD7 functions as a tumour suppressor through the regulation of PI3K activity.
Park, S. W. et al. BRD7 regulates XBP1s' activity and glucose homeostasis through its interaction with the regulatory subunits of PI3K. Cell Metab. 20, 73–84 (2014).
Kadoch, C. et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 45, 592–601 (2013). This proteomic and bioinformatic study identified new components of the mammalian SWI–SNF complex and mutations in them that are found in human cancers.
Fairbridge, N. A. et al. Cecr2 mutations causing exencephaly trigger misregulation of mesenchymal/ectodermal transcription factors. Birth Defects Res. A Clin. Mol. Teratol. 88, 619–625 (2010).
Banting, G. S. et al. CECR2, a protein involved in neurulation, forms a novel chromatin remodeling complex with SNF2L. Hum. Mol. Genet. 14, 513–524 (2005).
Bowser, R., Giambrone, A. & Davies, P. FAC1, a novel gene identified with the monoclonal antibody Alz50, is developmentally regulated in human brain. Dev. Neurosci. 17, 20–37 (1995).
Tallant, C. et al. Molecular basis of histone tail recognition by human TIP5 PHD finger and bromodomain of the chromatin remodeling complex NoRC. Structure 23, 80–92 (2015).
Collins, N. et al. An ACF1−ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nat. Genet. 32, 627–632 (2002).
Xiao, A. et al. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature 457, 57–62 (2009).
Zhou, Y. et al. Reversible acetylation of the chromatin remodelling complex NoRC is required for non-coding RNA-dependent silencing. Nat. Cell Biol. 11, 1010–1016 (2009).
Jones, M. H., Hamana, N., Nezu, J. & Shimane, M. A novel family of bromodomain genes. Genomics 63, 40–45 (2000).
Huang, H., Rambaldi, I., Daniels, E. & Featherstone, M. Expression of the WDR9 gene and protein products during mouse development. Dev. Dyn. 227, 608–614 (2003).
Philipps, D. L. et al. The dual bromodomain and WD repeat-containing mouse protein BRWD1 is required for normal spermiogenesis and the oocyte−embryo transition. Dev. Biol. 317, 72–82 (2008).
Pattabiraman, S. et al. Mouse BRWD1 is critical for spermatid postmeiotic transcription and female meiotic chromosome stability. J. Cell Biol. 208, 53–69 (2015).
Muller, P., Kuttenkeuler, D., Gesellchen, V., Zeidler, M. P. & Boutros, M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 436, 871–875 (2005).
Dancy, B. M. & Cole, P. A. Protein lysine acetylation by p300/CBP. Chem. Rev. 115, 2419–2452 (2015).
Yao, T. P. et al. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93, 361–372 (1998).
Tanaka, Y. et al. Extensive brain hemorrhage and embryonic lethality in a mouse null mutant of CREB-binding protein. Mech. Dev. 95, 133–145 (2000).
Kasper, L. H. et al. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol. Cell. Biol. 26, 789–809 (2006).
Nagy, Z. & Tora, L. Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 26, 5341–5357 (2007).
Krebs, A. R., Karmodiya, K., Lindahl-Allen, M., Struhl, K. & Tora, L. SAGA and ATAC histone acetyl transferase complexes regulate distinct sets of genes and ATAC defines a class of p300-independent enhancers. Mol. Cell 44, 410–423 (2011).
Maurice, T. et al. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology 33, 1584–1602 (2008).
Xu, W. et al. Loss of Gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nat. Genet. 26, 229–232 (2000).
Bu, P., Evrard, Y. A., Lozano, G. & Dent, S. Y. Loss of Gcn5 acetyltransferase activity leads to neural tube closure defects and exencephaly in mouse embryos. Mol. Cell. Biol. 27, 3405–3416 (2007).
Doyon, Y., Selleck, W., Lane, W. S., Tan, S. & Cote, J. Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol. Cell. Biol. 24, 1884–1896 (2004).
Altaf, M. et al. NuA4-dependent acetylation of nucleosomal histones H4 and H2A directly stimulates incorporation of H2A.Z by the SWR1 complex. J. Biol. Chem. 285, 15966–15977 (2010).
Obri, A. et al. ANP32E is a histone chaperone that removes H2A.Z from chromatin. Nature 505, 648–653 (2014).
Yang, X. J. MOZ and MORF acetyltransferases: molecular interaction, animal development and human disease. Biochim. Biophys. Acta 1853, 1818–1826 (2015).
Klein, B. J. et al. Bivalent interaction of the PZP domain of BRPF1 with the nucleosome impacts chromatin dynamics and acetylation. Nucleic Acids Res. 44, 472–484 (2016).
You, L., Chen, L., Penney, J., Miao, D. & Yang, X. J. Expression atlas of the multivalent epigenetic regulator Brpf1 and its requirement for survival of mouse embryos. Epigenetics 9, 860–872 (2014).
Mishima, Y. et al. The Hbo1−Brd1/Brpf2 complex is responsible for global acetylation of H3K14 and required for fetal liver erythropoiesis. Blood 118, 2443–2453 (2011).
Feng, Y. et al. BRPF3−HBO1 regulates replication origin activation and histone H3K14 acetylation. EMBO J. 35, 176–192 (2016).
Gregory, G. D. et al. Mammalian ASH1L is a histone methyltransferase that occupies the transcribed region of active genes. Mol. Cell. Biol. 27, 8466–8479 (2007).
Tanaka, Y. et al. Dual function of histone H3 lysine 36 methyltransferase ASH1 in regulation of Hox gene expression. PLoS ONE 6, e28171 (2011).
Rao, R. C. & Dou, Y. Hijacked in cancer: the KMT2 (MLL) family of methyltransferases. Nat. Rev. Cancer 15, 334–346 (2015).
Milne, T. A. et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell 10, 1107–1117 (2002).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010). This study reports the first potent and selective thienodiazepine compound that targets BET family BRDs in a NUT midline carcinoma model.
Nicodeme, E. et al. Suppression of inflammation by a synthetic histone mimic. Nature 468, 1119–1123 (2010). This study reports the first benzodiazepine compound that targets the BET family of BRDs and its use as an anti-inflammatory agent.
Wang, F. et al. Brd2 disruption in mice causes severe obesity without type 2 diabetes. Biochem. J. 425, 71–83 (2010).
Shang, E., Wang, X., Wen, D., Greenberg, D. A. & Wolgemuth, D. J. Double bromodomain-containing gene Brd2 is essential for embryonic development in mouse. Dev. Dyn. 238, 908–917 (2009).
Houzelstein, D. et al. Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol. Cell. Biol. 22, 3794–3802 (2002).
Lamonica, J. M. et al. Bromodomain protein Brd3 associates with acetylated GATA1 to promote its chromatin occupancy at erythroid target genes. Proc. Natl Acad. Sci. USA 108, E159–E168 (2011).
Stonestrom, A. J. et al. Functions of BET proteins in erythroid gene expression. Blood 125, 2825–2834 (2015).
Jang, M. K. et al. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19, 523–534 (2005).
Yang, Z. et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19, 535–545 (2005).
Gaucher, J. et al. Bromodomain-dependent stage-specific male genome programming by Brdt. EMBO J. 31, 3809–3820 (2012).
Shang, E., Nickerson, H. D., Wen, D., Wang, X. & Wolgemuth, D. J. The first bromodomain of Brdt, a testis-specific member of the BET sub-family of double-bromodomain-containing proteins, is essential for male germ cell differentiation. Development 134, 3507–3515 (2007).
Matzuk, M. M. et al. Small-molecule inhibition of BRDT for male contraception. Cell 150, 673–684 (2012).
Buchmann, A. M., Skaar, J. R. & DeCaprio, J. A. Activation of a DNA damage checkpoint response in a TAF1-defective cell line. Mol. Cell. Biol. 24, 5332–5339 (2004).
Kimura, J. et al. A functional genome-wide RNAi screen identifies TAF1 as a regulator for apoptosis in response to genotoxic stress. Nucleic Acids Res. 36, 5250–5259 (2008).
Sdelci, S. et al. Mapping the chemical chromatin reactivation landscape identifies BRD4−TAF1 cross-talk. Nat. Chem. Biol. 12, 504–510 (2016).
Wang, P. J. & Page, D. C. Functional substitution for TAFII250 by a retroposed homolog that is expressed in human spermatogenesis. Hum. Mol. Genet. 11, 2341–2346 (2002).
Gong, F. et al. Screen identifies bromodomain protein ZMYND8 in chromatin recognition of transcription-associated DNA damage that promotes homologous recombination. Genes Dev. 29, 197–211 (2015).
Shen, H. et al. Suppression of enhancer overactivation by a RACK7-histone demethylase complex. Cell 165, 331–342 (2016).
Wen, H. et al. ZMYND11 links histone H3.3K36me3 to transcription elongation and tumour suppression. Nature 508, 263–268 (2014).
Guo, R. et al. BS69/ZMYND11 reads and connects histone H3.3 lysine 36 trimethylation-decorated chromatin to regulated pre-mRNA processing. Mol. Cell 56, 298–310 (2014).
Zou, J. X., Revenko, A. S., Li, L. B., Gemo, A. T. & Chen, H. W. ANCCA, an estrogen-regulated AAA+ ATPase coactivator for ERα, is required for coregulator occupancy and chromatin modification. Proc. Natl Acad. Sci. USA 104, 18067–18072 (2007).
Ciro, M. et al. ATAD2 is a novel cofactor for MYC, overexpressed and amplified in aggressive tumors. Cancer Res. 69, 8491–8498 (2009).
Revenko, A. S., Kalashnikova, E. V., Gemo, A. T., Zou, J. X. & Chen, H. W. Chromatin loading of E2F−MLL complex by cancer-associated coregulator ANCCA via reading a specific histone mark. Mol. Cell. Biol. 30, 5260–5272 (2010).
Leachman, N. T., Brellier, F., Ferralli, J., Chiquet-Ehrismann, R. & Tucker, R. P. ATAD2B is a phylogenetically conserved nuclear protein expressed during neuronal differentiation and tumorigenesis. Dev. Growth Differ. 52, 747–755 (2010).
Morozumi, Y. et al. Atad2 is a generalist facilitator of chromatin dynamics in embryonic stem cells. J. Mol. Cell. Biol. 8, 349–362 (2016).
Malovannaya, A. et al. Analysis of the human endogenous coregulator complexome. Cell 145, 787–799 (2011). A large-scale affinity purification–mass spectrometry study that charts endogenous human co-regulator complexes.
Cammas, F. et al. Cell differentiation induces TIF1β association with centromeric heterochromatin via an HP1 interaction. J. Cell Sci. 115, 3439–3448 (2002).
Ivanov, A. V. et al. PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol. Cell 28, 823–837 (2007).
Zeng, L. et al. Structural insights into human KAP1 PHD finger-bromodomain and its role in gene silencing. Nat. Struct. Mol. Biol. 15, 626–633 (2008).
Tubbs, A. T. et al. KAP-1 promotes resection of broken DNA ends not protected by γ-H2AX and 53BP1 in G1-phase lymphocytes. Mol. Cell. Biol. 34, 2811–2821 (2014).
Dupont, S. et al. FAM/USP9x, a deubiquitinating enzyme essential for TGFβ signaling, controls Smad4 monoubiquitination. Cell 136, 123–135 (2009).
Agricola, E., Randall, R. A., Gaarenstroom, T., Dupont, S. & Hill, C. S. Recruitment of TIF1γ to chromatin via its PHD finger-bromodomain activates its ubiquitin ligase and transcriptional repressor activities. Mol. Cell 43, 85–96 (2011).
Kim, J. & Kaartinen, V. Generation of mice with a conditional allele for Trim33. Genesis 46, 329–333 (2008).
Khetchoumian, K. et al. TIF1δ, a novel HP1-interacting member of the transcriptional intermediary factor 1 (TIF1) family expressed by elongating spermatids. J. Biol. Chem. 279, 48329–48341 (2004).
Bloch, D. B. et al. Sp110 localizes to the PML−Sp100 nuclear body and may function as a nuclear hormone receptor transcriptional coactivator. Mol. Cell. Biol. 20, 6138–6146 (2000).
Wasylyk, C., Schlumberger, S. E., Criqui-Filipe, P. & Wasylyk, B. Sp100 interacts with ETS-1 and stimulates its transcriptional activity. Mol. Cell. Biol. 22, 2687–2702 (2002).
Yordy, J. S. et al. SP100 expression modulates ETS1 transcriptional activity and inhibits cell invasion. Oncogene 23, 6654–6665 (2004).
Podcheko, A. et al. Identification of a WD40 repeat-containing isoform of PHIP as a novel regulator of β-cell growth and survival. Mol. Cell. Biol. 27, 6484–6496 (2007).
Huether, R. et al. The landscape of somatic mutations in epigenetic regulators across 1,000 paediatric cancer genomes. Nat. Commun. 5, 3630 (2014). This large-scale study provided important insights into the mutational landscape of BRD-containing proteins across 1,000 paediatric cancer genomes.
Forbes, S. A. et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 43, D805–D811 (2015).
Cleary, S. P. et al. Identification of driver genes in hepatocellular carcinoma by exome sequencing. Hepatology 58, 1693–1702 (2013).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012).
Liu, J. et al. Genome and transcriptome sequencing of lung cancers reveal diverse mutational and splicing events. Genome Res. 22, 2315–2327 (2012).
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550 (2014).
Liu, L. et al. Identification of hallmarks of lung adenocarcinoma prognosis using whole genome sequencing. Oncotarget 6, 38016–38028 (2015).
Peifer, M. et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 44, 1104–1110 (2012).
Grunwald, C. et al. Expression of multiple epigenetically regulated cancer/germline genes in nonsmall cell lung cancer. Int. J. Cancer 118, 2522–2528 (2006).
Zheng, C. X. et al. Whole-exome sequencing to identify novel somatic mutations in squamous cell lung cancers. Int. J. Oncol. 43, 755–764 (2013).
Ho, A. S. et al. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 45, 791–798 (2013).
Lourdusamy, A., Rahman, R. & Grundy, R. G. Expression alterations define unique molecular characteristics of spinal ependymomas. Oncotarget 6, 19780–19791 (2015).
Odejide, O. et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood 123, 1293–1296 (2014).
Petrini, I. et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat. Genet. 46, 844–849 (2014).
Ojesina, A. I. et al. Landscape of genomic alterations in cervical carcinomas. Nature 506, 371–375 (2014).
Shang, P., Meng, F., Liu, Y. & Chen, X. Overexpression of ANCCA/ATAD2 in endometrial carcinoma and its correlation with tumor progression and poor prognosis. Tumor Biol. 36, 4479–4485 (2015).
Okosun, J. et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat. Genet. 46, 176–181 (2014).
Song, Y. et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509, 91–95 (2014).
Gao, Y. B. et al. Genetic landscape of esophageal squamous cell carcinoma. Nat. Genet. 46, 1097–1102 (2014).
Zhang, L. et al. Genomic analyses reveal mutational signatures and frequently altered genes in esophageal squamous cell carcinoma. Am. J. Hum. Genet. 96, 597–611 (2015).
Oh, H. R., An, C. H., Yoo, N. J. & Lee, S. H. Somatic mutations of amino acid metabolism-related genes in gastric and colorectal cancers and their regional heterogeneity — a short report. Cell. Oncol. (Dordr.) 37, 455–461 (2014).
Zhang, L. H. et al. TRIM24 promotes glioma progression and enhances chemoresistance through activation of the PI3K/Akt signaling pathway. Oncogene 34, 600–610 (2015).
Chen, Y. et al. TRIM66 overexpresssion contributes to osteosarcoma carcinogenesis and indicates poor survival outcome. Oncotarget 6, 23708–23719 (2015).
Kuroyanagi, J. et al. Zinc finger MYND-type containing 8 promotes tumour angiogenesis via induction of vascular endothelial growth factor-A expression. FEBS Lett. 588, 3409–3416 (2014).
Alekseyenko, A. A. et al. The oncogenic BRD4−NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 29, 1507–1523 (2015).
Andreasen, S., French, C. A., Josiassen, M., Hahn, C. H. & Kiss, K. NUT carcinoma of the sublingual gland. Head Neck Pathol. 10, 362–366 (2015).
Reynoird, N. et al. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J. 29, 2943–2952 (2010).
Pleasance, E. D. et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 463, 184–190 (2010).
Gocho, Y. et al. A novel recurrent EP300−ZNF384 gene fusion in B-cell precursor acute lymphoblastic leukemia. Leukemia 29, 2445–2448 (2015).
Marschalek, R. Systematic classification of mixed-lineage leukemia fusion partners predicts additional cancer pathways. Ann. Lab. Med. 36, 85–100 (2016).
Panagopoulos, I. et al. Fusion of ZMYND8 and RELA genes in acute erythroid leukemia. PLoS ONE 8, e63663 (2013).
Edgren, H. et al. Identification of fusion genes in breast cancer by paired-end RNA-sequencing. Genome Biol. 12, R6 (2011).
Kalyana-Sundaram, S. et al. Gene fusions associated with recurrent amplicons represent a class of passenger aberrations in breast cancer. Neoplasia 14, 702–708 (2012).
de Rooij, J. D. et al. Recurrent translocation t(10;17)(p15;q21) in minimally differentiated acute myeloid leukemia results in ZMYND11/MBTD1 fusion. Genes Chromosomes Cancer 55, 237–241 (2016).
Frattini, V. et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat. Genet. 45, 1141–1149 (2013).
Robinson, D. R. et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat. Med. 17, 1646–1651 (2011).
Asmann, Y. W. et al. Detection of redundant fusion transcripts as biomarkers or disease-specific therapeutic targets in breast cancer. Cancer Res. 72, 1921–1928 (2012).
Wu, W. J. et al. Prognostic relevance of BRD7 expression in colorectal carcinoma. Eur. J. Clin. Invest. 43, 131–140 (2013).
Park, Y. A. et al. Tumor suppressive effects of bromodomain-containing protein 7 (BRD7) in epithelial ovarian carcinoma. Clin. Cancer Res. 20, 565–575 (2014).
Herquel, B. et al. Transcription cofactors TRIM24, TRIM28, and TRIM33 associate to form regulatory complexes that suppress murine hepatocellular carcinoma. Proc. Natl Acad. Sci. USA 108, 8212–8217 (2011).
Xue, J. et al. Tumour suppressor TRIM33 targets nuclear β-catenin degradation. Nat. Commun. 6, 6156 (2015).
Varela, I. et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469, 539–542 (2011). This large-scale study demonstrated the power of the whole-exome sequencing technique to identify mutations in BRD-containing proteins.
Jiao, Y. et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat. Genet. 45, 1470–1473 (2013).
Wilson, B. G. & Roberts, C. W. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer 11, 481–492 (2011).
Egelhofer, T. A. et al. An assessment of histone-modification antibody quality. Nat. Struct. Mol. Biol. 18, 91–93 (2011).
Rothbart, S. B. et al. An interactive database for the assessment of histone antibody specificity. Mol. Cell 59, 502–511 (2015).
Zhang, G. et al. Down-regulation of NF-κB transcriptional activity in HIV-associated kidney disease by BRD4 inhibition. J. Biol. Chem. 287, 28840–28851 (2012).
Li, A. G. et al. An acetylation switch in p53 mediates holo-TFIID recruitment. Mol. Cell 28, 408–421 (2007).
Ciceri, P. et al. Dual kinase-bromodomain inhibitors for rationally designed polypharmacology. Nat. Chem. Biol. 10, 305–312 (2014).
Martin, M. P., Olesen, S. H., Georg, G. I. & Schonbrunn, E. Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains. ACS Chem. Biol. 8, 2360–2365 (2013).
Dittmann, A. et al. The commonly used PI3-kinase probe LY294002 is an inhibitor of BET bromodomains. ACS Chem. Biol. 9, 495–502 (2014).
Ember, S. W. et al. Acetyl-lysine binding site of bromodomain-containing protein 4 (BRD4) interacts with diverse kinase inhibitors. ACS Chem. Biol. 9, 1160–1171 (2014).
Allen, B. K. et al. Large-scale computational screening identifies first in class multitarget inhibitor of EGFR kinase and BRD4. Sci. Rep. 5, 16924 (2015).
Kurimchak, A. M. et al. Resistance to BET bromodomain inhibitors is mediated by kinome reprogramming in ovarian cancer. Cell Rep. 16, 1273–1286 (2016).
Dawson, M. A. et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 478, 529–533 (2011).
Stathis, A. et al. Clinical response of carcinomas harboring the BRD4−NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 6, 492–500 (2016).
Bolden, J. E. et al. Inducible in vivo silencing of Brd4 identifies potential toxicities of sustained BET protein inhibition. Cell Rep. 8, 1919–1929 (2014).
Di Micco, R. et al. Control of embryonic stem cell identity by BRD4-dependent transcriptional elongation of super-enhancer-associated pluripotency genes. Cell Rep. 9, 234–247 (2014). References 182 and 183 raise concerns about the potential toxicity of inhibiting BET proteins.
Korb, E., Herre, M., Zucker-Scharff, I., Darnell, R. B. & Allis, C. D. BET protein Brd4 activates transcription in neurons and BET inhibitor Jq1 blocks memory in mice. Nat. Neurosci. 18, 1464–1473 (2015).
Fong, C. Y. et al. BET inhibitor resistance emerges from leukaemia stem cells. Nature 525, 538–542 (2015).
Rathert, P. et al. Transcriptional plasticity promotes primary and acquired resistance to BET inhibition. Nature 525, 543–547 (2015).
Shu, S. et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature 529, 413–417 (2016).
Togel, L. et al. Dual targeting of bromodomain and extra-terminal domain proteins, and WNT or MAPK signaling, inhibits c-MYC expression and proliferation of colorectal cancer cells. Mol. Cancer Ther. 15, 1217–1226 (2016).
Acknowledgements
The authors apologize to all researchers whose important contributions could not be acknowledged owing to space limitations. The authors are grateful for the support for their research received from the Ludwig Institute for Cancer Research and the Structural Genomics Consortium (SGC), which is a registered charity (number 1097737) that receives funds from AbbVie, Bayer Pharma AG, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genome Canada, Innovative Medicines Initiative (EU/EFPIA) [ULTRA-DD grant no. 115766], Janssen, Merck & Co., Novartis Pharma AG, Ontario Ministry of Economic Development and Innovation, Pfizer, São Paulo Research Foundation-FAPESP, Takeda and the Wellcome Trust (092809/Z/10/Z). T.F. is supported by a Uehara Memorial Foundation Fellowship (201330102). P.F. is supported by a Wellcome Trust Career Development Fellowship (095751/Z/11/Z).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Role(s) of BRD-containing proteins in cellular homeostasis (PDF 258 kb)
Supplementary information S2 (figure)
Structural Classification of the human BRD family. (PDF 3766 kb)
Supplementary information S3 (table)
Structures of BRD/peptide complexes (PDF 185 kb)
Supplementary information S4 (table)
Role(s) of BRD-containing proteins in cancer (mutations, expression, translocations) (PDF 307 kb)
Supplementary information S5 (table)
Mutation load of BRD modules. (PDF 2511 kb)
Supplementary information
Supplementary information S6 (figure) (XLSX 49 kb)
Supplementary information S7 (table)
Available Chemical Tools targeting BRD modules with low nM activity (PDF 179 kb)
Supplementary information S8 (table)
Bromodomain Inhibitors in Clinical Development (data from www.clinicaltrials.gov) (PDF 162 kb)
Related links
Related links
FURTHER INFORMATION
Glossary
- YEATS domains
-
Protein interaction domains with an immunoglobulin-like fold that can bind to acetylated and crotonylated Lys residues.
- Transcriptional co-regulators
-
Proteins that repress or stimulate transcription via the modulation of the activity of transcription factors through various mechanisms.
- Histone code
-
The epigenetic combination of histone modifications that affect transcription of genes in a hereditary fashion.
- Epitopes
-
The part of a protein that can be recognized by a protein interaction domain. In the context of antibodies, it is the part of an antigen molecule to which an antibody binds.
- Epithelial–mesenchymal transition
-
(EMT). The process by which epithelial cells lose polarity and adhesion and acquire the invasive and migratory properties of mesenchymal cells.
- Epicardium
-
Connective tissue that forms a protective layer around the heart muscle.
- Centromeres
-
Portions of the chromosome that link the sister chromatids. Cohesion of the centromeres is achieved by associating with the protein cohesin, which is subsequently modified in order to keep the two sister chromatids together.
- Homeobox genes
-
(HOX genes). A group of related homeotic genes that control the body plan of embryos along the head–tail axis.
- Enhancer
-
A short region of DNA that provides a docking site for transcriptional activators that increases the likelihood of transcription of particular genes.
- RING domain
-
(Really interesting new gene domain). Zinc finger domain containing a Cys3HisCys4 motif that binds two zinc ions.
- NUT midline carcinomas
-
Aggressive epithelial cancers that typically arise in organs in the midline of the body and result from a fusion of the NUT gene with BRD3, BRD4 or nuclear SET domain-containing gene 3 (NSD3).
- Mediator
-
Large multiprotein complex that acts as a transcriptional co-activator in eukaryotes and binds to the C-terminal domain of RNAPII, which provides a bridge between the polymerase and transcription factors.
Rights and permissions
About this article
Cite this article
Fujisawa, T., Filippakopoulos, P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat Rev Mol Cell Biol 18, 246–262 (2017). https://doi.org/10.1038/nrm.2016.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2016.143
This article is cited by
-
Targeting bromodomain-containing proteins: research advances of drug discovery
Molecular Biomedicine (2023)
-
Male contraception: narrative review of ongoing research
Basic and Clinical Andrology (2023)
-
A complex network of transcription factors and epigenetic regulators involved in bovine leukemia virus transcriptional regulation
Retrovirology (2023)
-
Targeted protein degradation reveals BET bromodomains as the cellular target of Hedgehog pathway inhibitor-1
Nature Communications (2023)
-
Crystal structure of [1,2,4]triazolo[4,3-b]pyridazine derivatives as BRD4 bromodomain inhibitors and structure–activity relationship study
Scientific Reports (2023)