Key Points
-
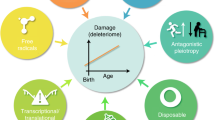
Mitochondrial dysfunction and genomic instability are two hallmarks of ageing.
-
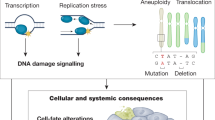
Nuclear DNA damage accumulates with ageing and contributes to ageing-associated diseases. Signalling from the nucleus to mitochondria (NM signalling) has a crucial role in regulating mitochondrial function and ageing.
-
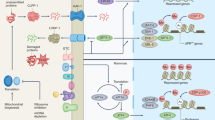
Three major NM signalling pathways link DNA damage to mitochondrial dysfunction: nuclear DNA repair and mitochondrial homeostasis; nuclear DNA damage-induced metabolic pathways; and regulation of mitophagy–apoptosis crosstalk by nuclear DNA repair-associated proteins.
-
NM signalling pathways can be targeted pharmacologically to promote healthy ageing and to treat age-associated diseases.
Abstract
Mitochondrial dysfunction is a hallmark of ageing, and mitochondrial maintenance may lead to increased healthspan. Emerging evidence suggests a crucial role for signalling from the nucleus to mitochondria (NM signalling) in regulating mitochondrial function and ageing. An important initiator of NM signalling is nuclear DNA damage, which accumulates with age and may contribute to the development of age-associated diseases. DNA damage-dependent NM signalling constitutes a network that includes nuclear sirtuins and controls genomic stability and mitochondrial integrity. Pharmacological modulation of NM signalling is a promising novel approach for the prevention and treatment of age-associated diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
24 March 2016
The title of Figure 2 was incorrectly phrased in the original HTML and PDF versions of this article. This has been corrected to "PARP1–NAD+–SIRT1-mediated nuclear DNA damage to mitochondria signalling".
References
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015).
Wallace, D. C. Mitochondrial DNA variation in human radiation and disease. Cell 163, 33–38 (2015).
Scheibye-Knudsen, M. et al. Protecting the mitochondrial powerhouse. Trends Cell Biol. 25, 158–170. (2014).
Maynard, S., Fang, E. F., Scheibye-Knudsen, M., Croteau, D. L. & Bohr, V. A. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb. Perspect. Med. 5, a025130 (2015).
Youle, R. J. & van der Bliek, A. M. Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 (2012).
Randow, F. & Youle, R. J. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe 15, 403–411 (2014).
Wallace, D. C. Mitochondria and cancer. Nat. Rev. Cancer 12, 685–698 (2012).
Coskun, P. et al. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim. Biophys. Acta 1820, 553–564 (2012).
Mattson, M. P. Pathways towards and away from Alzheimer's disease. Nature 430, 631–639 (2004).
Wallace, D. C. Mitochondrial diseases in man and mouse. Science 283, 1482–1488 (1999).
Palikaras, K., Lionaki, E. & Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521, 525–528 (2015). Provides evidence for a pivotal role of mitophagy in healthspan and lifespan in C. elegans.
Fang, E. F. et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD+/SIRT1 reduction. Cell 157, 882–896 (2014).
Menzies, F. M., Fleming, A. & Rubinsztein, D. C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 16, 345–357 (2015).
Petersen, K. F. et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 (2003).
Conley, K. E., Jubrias, S. A. & Esselman, P. C. Oxidative capacity and ageing in human muscle. J. Physiol. 526, 203–210 (2000).
Mouchiroud, L. et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 (2013). Shows that the UPRmt contributes to SIR2.1-related longevity in C. elegans.
Hey-Mogensen, M. et al. A novel method for determining human ex vivo submaximal skeletal muscle mitochondrial function. J. Physiol. 593, 3991–4010 (2015).
Capel, F. et al. Due to reverse electron transfer, mitochondrial H2O2 release increases with age in human vastus lateralis muscle although oxidative capacity is preserved. Mech. Ageing Dev. 126, 505–511 (2005).
Maynard, S. et al. Relationships between human vitality and mitochondrial respiratory parameters, reactive oxygen species production and dNTP levels in peripheral blood mononuclear cells. Aging 5, 850–864 (2013).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Scheibye-Knudsen, M. et al. Cockayne syndrome group B protein prevents the accumulation of damaged mitochondria by promoting mitochondrial autophagy. J. Exp. Med. 209, 855–869 (2012).
Scheibye-Knudsen, M., Fang, E. F., Croteau, D. L. & Bohr, V. A. Contribution of defective mitophagy to the neurodegeneration in DNA repair-deficient disorders. Autophagy 10, 1468–1469 (2014).
Shiloh, Y. & Ziv, Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 14, 197–210 (2013).
Houtkooper, R. H. et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497, 451–457 (2013).
Mohrin, M. et al. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 347, 1374–1377 (2015).
Pellegrino, M. W., Nargund, A. M. & Haynes, C. M. Signaling the mitochondrial unfolded protein response. Biochim. Biophys. Acta 1833, 410–416 (2013).
Canto, C., Menzies, K. J. & Auwerx, J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22, 31–53 (2015).
Michikawa, Y., Mazzucchelli, F., Bresolin, N., Scarlato, G. & Attardi, G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science 286, 774–779 (1999).
Croteau, D. L., Popuri, V., Opresko, P. L. & Bohr, V. A. Human RecQ helicases in DNA repair, recombination, and replication. Annu. Rev. Biochem. 83, 519–552 (2014).
Yang, H. et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130, 1095–1107 (2007).
Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 350, 1208–1213 (2015).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Chalkiadaki, A. & Guarente, L. The multifaceted functions of sirtuins in cancer. Nat. Rev. Cancer 15, 608–624 (2015).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000). The first evidence linking NAD+ to a sirtuin.
Scheibye-Knudsen, M. et al. A high fat diet and NAD+ rescue premature aging in Cockayne syndrome. Cell Metab. 20, 840–855 (2014). Together with reference 12, establishes a causative link from DNA damage to mitochondrial dysfunction and premature ageing.
Gibson, B. A. & Kraus, W. L. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 13, 411–424 (2012).
Eliasson, M. J. et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 3, 1089–1095 (1997).
Virag, L. & Szabo, C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 54, 375–429 (2002).
Liu, D., Gharavi, R., Pitta, M., Gleichmann, M. & Mattson, M. P. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 11, 28–42 (2009).
Pacher, P. & Szabo, C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am. J. Pathol. 173, 2–13 (2008).
Bai, P. et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 (2011).
DiGiovanna, J. J. & Kraemer, K. H. Shining a light on xeroderma pigmentosum. J. Invest. Dermatol. 132, 785–796 (2012).
Cleaver, J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature 218, 652–656 (1968).
Lindenbaum, Y. et al. Xeroderma pigmentosum/Cockayne syndrome complex: first neuropathological study and review of eight other cases. Eur. J. Paediatr. Neurol. 5, 225–242 (2001).
Canto, C. et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009).
Gomes, A. P. et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638 (2013). Demonstrates a role for NAD+ in the regulation of nuclear–mitochondrial communication.
Dobbin, M. M. et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat. Neurosci. 16, 1008–1015 (2013).
Cohen, H. Y. et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305, 390–392 (2004).
Li, K. et al. Regulation of WRN protein cellular localization and enzymatic activities by SIRT1-mediated deacetylation. J. Biol. Chem. 283, 7590–7598 (2008).
Uhl, M. et al. Role of SIRT1 in homologous recombination. DNA Repair 9, 383–393 (2010).
Yamamori, T. et al. SIRT1 deacetylates APE1 and regulates cellular base excision repair. Nucleic Acids Res. 38, 832–845 (2010).
Madabushi, A., Hwang, B. J., Jin, J. & Lu, A. L. Histone deacetylase SIRT1 modulates and deacetylates DNA base excision repair enzyme thymine DNA glycosylase. Biochem. J. 456, 89–98 (2013).
Fan, W. & Luo, J. SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Mol. Cell 39, 247–258 (2010).
Kanfi, Y. et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature 483, 218–221 (2012).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Sebastian, C. et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 151, 1185–1199 (2012).
Liu, G. H. et al. Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature 472, 221–225 (2011).
Ghosh, S., Liu, B., Wang, Y., Hao, Q. & Zhou, Z. Lamin A is an endogenous SIRT6 activator and promotes SIRT6-mediated DNA repair. Cell Rep. 13, 1396–1406 (2015).
Zhong, L. et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell 140, 280–293 (2010).
Kaidi, A., Weinert, B. T., Choudhary, C. & Jackson, S. P. Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science 329, 1348–1353 (2010).
Mao, Z. et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science 332, 1443–1446 (2011).
Michishita, E. et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452, 492–496 (2008).
McCord, R. A. et al. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging 1, 109–121 (2009).
Toiber, D. et al. SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Mol. Cell 51, 454–468 (2013).
Van Meter, M. et al. SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat. Commun. 5, 5011 (2014).
Barber, M. F. et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 487, 114–118 (2012).
Shin, J. et al. SIRT7 represses Myc activity to suppress ER stress and prevent fatty liver disease. Cell Rep. 5, 654–665 (2013).
Ryu, D. et al. A SIRT7-dependent acetylation switch of GABPβ1 controls mitochondrial function. Cell Metab. 20, 856–869 (2014). Provides evidence that SIRT7 regulates mitochondrial function.
Someya, S. et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143, 802–812 (2010).
Brown, K. D. et al. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 20, 1059–1068 (2014).
Cheng, A. et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 23, 128–142 (2016).
Hirschey, M. D. et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 (2010).
Jeong, S. M. et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 23, 450–463 (2013).
Kim, H. S. et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 12, 224–236 (2010).
Malik, S. et al. SIRT7 inactivation reverses metastatic phenotypes in epithelial and mesenchymal tumors. Sci. Rep. 5, 9841 (2015).
Lee, N. et al. Comparative interactomes of SIRT6 and SIRT7: implication of functional links to aging. Proteomics 14, 1610–1622 (2014).
Woods, C. G. & Taylor, A. M. Ataxia telangiectasia in the British Isles: the clinical and laboratory features of 70 affected individuals. Q. J. Med. 82, 169–179 (1992).
Merideth, M. A. et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N. Engl. J. Med. 358, 592–604 (2008).
Dahl, A. K. et al. Body mass index, change in body mass index, and survival in old and very old persons. J. Am. Geriatr. Soc. 61, 512–518 (2013).
Valentin-Vega, Y. A. et al. Mitochondrial dysfunction in ataxia-telangiectasia. Blood 119, 1490–1500 (2012).
Williamson, D. H., Lund, P. & Krebs, H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 103, 514–527 (1967).
Ross, J. M. et al. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc. Natl Acad. Sci. USA 107, 20087–20092 (2010).
Sutendra, G. et al. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell 158, 84–97 (2014).
Mone, M. J. et al. Local UV-induced DNA damage in cell nuclei results in local transcription inhibition. EMBO Rep. 2, 1013–1017 (2001).
Shanbhag, N. M., Rafalska-Metcalf, I. U., Balane-Bolivar, C., Janicki, S. M. & Greenberg, R. A. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell 141, 970–981 (2010).
Longo, V. D. & Mattson, M. P. Fasting: molecular mechanisms and clinical applications. Cell Metab. 19, 181–192 (2014).
Tripathi, D. N. et al. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc. Natl Acad. Sci. USA 110, E2950–E2957 (2013).
Budanov, A. V. & Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134, 451–460 (2008).
Egan, D. F. et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 (2011).
Marino, G., Niso-Santano, M., Baehrecke, E. H. & Kroemer, G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15, 81–94 (2014).
Youle, R. J. & Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 (2008).
Rubinsztein, D. C., Marino, G. & Kroemer, G. Autophagy and aging. Cell 146, 682–695 (2011).
Allen, G. F., Toth, R., James, J. & Ganley, I. G. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 14, 1127–1135 (2013).
Chu, C. T. et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 15, 1197–1205 (2013).
Lazarou, M. et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314 (2015).
Pickrell, A. M. & Youle, R. J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85, 257–273 (2015).
Biton, S. & Ashkenazi, A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-α feedforward signaling. Cell 145, 92–103 (2011).
Picco, V. & Pages, G. Linking JNK activity to the DNA damage response. Genes Cancer 4, 360–368 (2013).
Luo, S. et al. Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol. Cell 47, 359–370 (2012).
Maryanovich, M. et al. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nat. Cell Biol. 14, 535–541 (2012).
Mercer, J. R. et al. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndrome. Circ. Res. 107, 1021–1031 (2010).
Scheibye-Knudsen, M., Scheibye-Alsing, K., Canugovi, C., Croteau, D. L. & Bohr, V. A. A novel diagnostic tool reveals mitochondrial pathology in human diseases and aging. Aging 5, 192–208 (2013).
Oda, K. et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102, 849–862 (2000).
Murray-Zmijewski, F., Slee, E. A. & Lu, X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat. Rev. Mol. Cell Biol. 9, 702–712 (2008).
Hoshino, A. et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 4, 2308 (2013).
Crighton, D. et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 126, 121–134 (2006).
Xie, X., Le, L., Fan, Y., Lv, L. & Zhang, J. Autophagy is induced through the ROS-TP53-DRAM1 pathway in response to mitochondrial protein synthesis inhibition. Autophagy 8, 1071–1084 (2012).
Poyurovsky, M. V. & Prives, C. P53 and aging: a fresh look at an old paradigm. Aging 2, 380–382 (2010).
Matheu, A. et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375–379 (2007).
Pinkston, J. M., Garigan, D., Hansen, M. & Kenyon, C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science 313, 971–975 (2006).
Vaziri, H. et al. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001).
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001).
Sampaio-Marques, B. et al. SNCA (α-synuclein)-induced toxicity in yeast cells is dependent on sirtuin 2 (Sir2)-mediated mitophagy. Autophagy 8, 1494–1509 (2012).
Huang, R. et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 57, 456–466 (2015).
Price, N. L. et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15, 675–690 (2012).
Longo, V. D. et al. Interventions to slow aging in humans: are we ready? Aging Cell 14, 497–510 (2015).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02300740, (2014).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02303483, (2014).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02191462, (2014).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02678611, (2016).
Wang, G. et al. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 158, 1324–1334 (2014).
Hubbard, B. P. et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339, 1216–1219 (2013).
Sinclair, D. A. & Guarente, L. Small-molecule allosteric activators of sirtuins. Annu. Rev. Pharmacol. Toxicol. 54, 363–380 (2014).
van der Meer, A. J. et al. The selective sirtuin 1 activator SRT2104 reduces endotoxin-induced cytokine release and coagulation activation in humans. Crit. Care Med. 43, e199–202 (2015).
Timmers, S. et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 14, 612–622 (2011).
Kashiwaya, Y. et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol. Aging 34, 1530–1539 (2013).
Edwards, C. et al. d-β-hydroxybutyrate extends lifespan in C. elegans. Aging 6, 621–644 (2014).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Ravikumar, B. et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 36, 585–595 (2004). Provides the first evidence that compromised autophagy contributes to the pathology of the neurodegenerative disorder Huntington disease.
Leslie, M. A putative antiaging drug takes a step from mice to men. Science 342, 789 (2013).
Dai, D. F. et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, 529–539 (2014).
Soefje, S. A., Karnad, A. & Brenner, A. J. Common toxicities of mammalian target of rapamycin inhibitors. Target Oncol. 6, 125–129 (2011).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009).
Pavel, M. & Rubinsztein, D. C. in Antitumor Potential and Other Emerging Medicinal Properties of Natural Compounds (eds Fang, E. F. & Ng, T. B.) 227–238 (Springer, 2013).
Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011).
Saretzki, G. Extra-telomeric functions of human telomerase: cancer, mitochondria and oxidative stress. Curr. Pharm. Des. 20, 6386–6403 (2014).
Chen, L. Y. et al. Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Mol. Cell 47, 839–850 (2012).
Mangerich, A. & Burkle, A. Pleiotropic cellular functions of PARP1 in longevity and aging: genome maintenance meets inflammation. Oxid. Med. Cell. Longev. 2012, 321653 (2012).
Grube, K. & Burkle, A. Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc. Natl Acad. Sci. USA 89, 11759–11763 (1992).
Luna, A., Aladjem, M. I. & Kohn, K. W. SIRT1/PARP1 crosstalk: connecting DNA damage and metabolism. Genome Integr. 4, 6 (2013).
Hoeijmakers, J. H. DNA damage, aging, and cancer. N. Engl. J. Med. 361, 1475–1485 (2009).
Cleaver, J. E., Lam, E. T. & Revet, I. Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity. Nat. Rev. Genet. 10, 756–768 (2009).
Hanawalt, P. C. & Spivak, G. Transcription-coupled DNA repair: two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 9, 958–970 (2008).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Lakshmipathy, U. & Campbell, C. Double strand break rejoining by mammalian mitochondrial extracts. Nucleic Acids Res. 27, 1198–1204 (1999).
Alexeyev, M., Shokolenko, I., Wilson, G. & LeDoux, S. The maintenance of mitochondrial DNA integrity — critical analysis and update. Cold Spring Harb. Perspect. Biol. 5, a012641 (2013).
Ding, L. & Liu, Y. Borrowing nuclear DNA helicases to protect mitochondrial DNA. Int. J. Mol. Sci. 16, 10870–10887 (2015).
Croteau, D. L. et al. RECQL4 localizes to mitochondria and preserves mitochondrial DNA integrity. Aging Cell 11, 456–466 (2012).
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000).
Dang, W. et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 459, 802–807 (2009).
Viswanathan, M. & Guarente, L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature 477, E1–E2 (2011).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001).
Burnett, C. et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 477, 482–485 (2011).
Oberdoerffer, P. et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135, 907–918 (2008).
Finley, L. W. & Haigis, M. C. Metabolic regulation by SIRT3: implications for tumorigenesis. Trends Mol. Med. 18, 516–523 (2012).
Acknowledgements
The authors acknowledge the valuable work of the many investigators whose published articles they were unable to cite owing to space limitations. They thank Prabhat Khadka and Anne Tseng for critical reading of the manuscript. This research was supported entirely by the Intramural Research Program of the US National Institutes of Health (NIH) National Institute on Ageing (NIA). K.F.C. was supported by the Department of Veterans Affairs (Merit Award), research awards from the Glenn Foundation for Medical Research and the NIH/NIA (R56AG050997).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The Bohr laboratory has CRADA arrangements with ChromaDex and GlaxoSmithKline.
Related links
Glossary
- Cockayne syndrome
-
A rare accelerated-ageing disease with progressive neurodegeneration, caused by mutations in genes encoding two DNA repair proteins, CSA and CSB.
- Xeroderma pigmentosum
-
A rare autosomal-recessive disorder characterized by severe sun sensitivity and skin cancer, associated with mutation of genes encoding a group of DNA repair proteins, XPA to XPG.
- Ataxia telangiectasia
-
A genomic instability disease with progressive cerebellar neurodegeneration caused by mutation of the ataxia telangiectasia mutated (ATM) gene, encoding the kinase ATM, which is a master regulator of DNA damage processing.
- Preiss–Handler pathway
-
A NAD+ biosynthetic process that consumes dietary nicotinic acid.
- Salvage pathway
-
A NAD+ biosynthetic pathway that uses nicotinamide to generate nicotinamide mononucleotide (NMN), which is then transformed into NAD+.
- Reactive oxygen species
-
(ROS). By-products of cellular metabolism, which at low levels provide health benefits, whereas at high levels they become increasingly noxious, with broad pathological consequences.
- Ataxia telangiectasia mutated
-
(ATM). A 350 kDa Ser/Thr protein kinase that is required for activation of the DNA damage response to double-strand breaks through phosphorylation of >700 downstream DNA repair proteins.
- Hepatosteatosis
-
Also known as hepatic steatosis (fatty liver). A common liver abnormality, in which patients have excessive accumulation of triglycerides (lipid droplets) in the liver.
- Apurinic and apyrimidinic sites
-
(AP sites; also known as abasic sites). Sites of DNA sugar without a base, which is a common DNA lesion and is typically repaired by DNA base excision repair through sugar cleavage by AP endonuclease 1 (APE1).
- 8-oxo-dGuo
-
(8-oxo-7,8-dihydro-2′- deoxyguanosine). An oxidative DNA lesion, which can be repaired by DNA base excision repair.
- NAD+/NADH ratio
-
NAD exists in cells in both oxidized (NAD+) and reduced (NADH) forms. The ratio of NAD+ and NADH regulates many cellular processes, including energy metabolism and mitochondrial functions.
- Ketogenic diet
-
A diet that is high-fat, high-protein and low-carbohydrate.
- Stress response
-
A cellular response to certain types of stress, such as caloric restriction or increase in reactive oxygen species, in which different stress-counteracting pathways are upregulated.
- Autophagosome
-
A key structure of autophagy, an autophagosome is a spherical, double-membrane vesicle that sequesters cytoplasmic contents for degradation.
- Rapamycin
-
A natural metabolite from the bacterium Streptomyces hygroscopicus, which inhibits mTOR and extends lifespan in species from yeast to fruit flies and mice.
Rights and permissions
About this article
Cite this article
Fang, E., Scheibye-Knudsen, M., Chua, K. et al. Nuclear DNA damage signalling to mitochondria in ageing. Nat Rev Mol Cell Biol 17, 308–321 (2016). https://doi.org/10.1038/nrm.2016.14
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2016.14
This article is cited by
-
The fusion of multi-omics profile and multimodal EEG data contributes to the personalized diagnostic strategy for neurocognitive disorders
Microbiome (2024)
-
Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases
Translational Neurodegeneration (2023)
-
Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells
Journal of Translational Medicine (2023)
-
Fatty acid oxidation facilitates DNA double-strand break repair by promoting PARP1 acetylation
Cell Death & Disease (2023)
-
Metabolic landscape in cardiac aging: insights into molecular biology and therapeutic implications
Signal Transduction and Targeted Therapy (2023)