Key Points
-
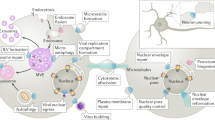
Endosomal sorting complex required for transport (ESCRT) proteins carry out scission of membrane necks with a topology (or sidedness) opposite to that of the better-understood process carried out by coat proteins, dynamin and BAR (Bin, amphiphysin and Rvs) domain proteins.
-
ESCRT-mediated reverse-topology membrane scission is initiated by two upstream branches: the first comprising ESCRT-I and ESCRT-II, and the second comprising ALIX.
-
The ESCRT-III protein family has 12 different subunits in humans. ESCRT-III monomers are about 200 amino acids in length and have open and closed conformations.
-
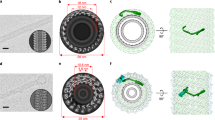
ESCRT-III proteins can assemble into flat spirals, helical tubes or conical funnels.
-
ESCRT-III assemblies are taken apart by the AAA+ ATPase vacuolar protein sorting-associated 4 (VPS4), which unfolds ESCRT-III monomers and threads them through a central pore of the VPS4 hexamer.
-
It is currently unresolved whether scission is mediated by the drawing-together of membrane necks by a tapered dome, buckling by the mechanical spring-like action of curved ESCRT filaments or some other means.
Abstract
The narrow membrane necks formed during viral, exosomal and intra-endosomal budding from membranes, as well as during cytokinesis and related processes, have interiors that are contiguous with the cytosol. Severing these necks involves action from the opposite face of the membrane as occurs during the well-characterized formation of coated vesicles. This 'reverse' (or 'inverse')-topology membrane scission is carried out by the endosomal sorting complex required for transport (ESCRT) proteins, which form filaments, flat spirals, tubes and conical funnels that are thought to direct membrane remodelling and scission. Their assembly, and their disassembly by the ATPase vacuolar protein sorting-associated 4 (VPS4) have been intensively studied, but the mechanism of scission has been elusive. New insights from cryo-electron microscopy and various types of spectroscopy may finally be close to rectifying this situation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Votteler, J. & Sundquist, W. I. Virus budding and the ESCRT pathway. Cell Host Microbe 14, 232–241 (2013).
Martin-Serrano, J. & Neil, S. J. D. Host factors involved in retroviral budding and release. Nat. Rev. Microbiol. 9, 519–531 (2011).
Olmos, Y. & Carlton, J. G. The ESCRT machinery: new roles at new holes. Curr. Opin. Cell Biol. 38, 1–11 (2016).
Campsteijn, C., Vietri, M. & Stenmark, H. Novel ESCRT functions in cell biology: spiraling out of control? Curr. Opin. Cell Biol. 41, 1–8 (2016).
Hurley, J. H. ESCRTs are everywhere. EMBO J. 34, 2398–2407 (2015).
Shields, S. B. & Piper, R. C. How ubiquitin functions with ESCRTs. Traffic 12, 1307–1317 (2011).
Katzmann, D. J., Babst, M. & Emr, S. D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106, 145–155 (2001).
Kostelansky, M. S. et al. Molecular architecture and functional model of the complete yeast ESCRT-I heterotetramer. Cell 129, 485–498 (2007).
Babst, M., Katzmann, D. J., Snyder, W. B., Wendland, B. & Emr, S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3, 283–289 (2002).
Hierro, A. et al. Structure of the ESCRT-II endosomal trafficking complex. Nature 431, 221–225 (2004).
Teo, H., Perisic, O., Gonzalez, B. & Williams, R. L. ESCRT-II, an endosome-associated complex required for protein sorting: Crystal structure and interactions with ESCRT-III and membranes. Dev. Cell 7, 559–569 (2004).
Im, Y. J. & Hurley, J. H. Integrated structural model and membrane targeting mechanism of the human ESCRT-II complex. Dev. Cell 14, 902–913 (2008).
Kostelansky, M. S. et al. Structural and functional organization of the ESCRT-I trafficking complex. Cell 125, 113–126 (2006).
Gill, D. J. et al. Structural insight into the ESCRT-I/-II link and its role in MVB trafficking. EMBO J. 26, 600–612 (2007).
Boura, E. et al. Solution structure of the ESCRT-I and -II supercomplex: Implications for membrane budding and scission. Structure 20, 874–886 (2012).
Wollert, T. & Hurley, J. H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 464, 864–869 (2010).
Carlson, L.-A. & Hurley, J. H. In vitro reconstitution of the ordered assembly of the endosomal sorting complex required for transport at membrane-bound HIV-1 Gag clusters. Proc. Natl Acad. Sci. USA 109, 16928–16933 (2012).
Morita, E. et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 26, 4215–4227 (2007).
Morita, E. et al. ESCRT-III protein requirements for HIV-1 budding. Cell Host Microbe 9, 235–242 (2011).
Goliand, I., Nachmias, D., Gershony, O. & Elia, N. Inhibition of ESCRT-II–CHMP6 interactions impedes cytokinetic abscission and leads to cell death. Mol. Biol. Cell 25, 3740–3748 (2014).
Meng, B., Ip, N. C. Y., Prestwood, L. J., Abbink, T. E. M. & Lever, A. M. L. Evidence that the endosomal sorting complex required for transport-II (ESCRT-II) is required for efficient human immunodeficiency virus-1 (HIV-1) production. Retrovirology 12, 72 (2015).
Christ, L. et al. ALIX and ESCRT-I/II function as parallel ESCRT-III recruiters in cytokinetic abscission. J. Cell Biol. 212, 499–513 (2016).
Tang, S. et al. ESCRT-III activation by parallel action of ESCRT-I/II and ESCRT-0/Bro1 during MVB biogenesis. eLife http://dx.doi.org/10.7554/eLife.15507 (2016).
Cashikar, A. G. et al. Structure of cellular ESCRT-III spirals and their relationship to HIV budding. eLife http://dx.doi.org/10.7554/eLife.02184 (2014). Uses EM of ESCRT-III at HIV-1 Gag budding sites and reveals a funnel that nucleates at a narrow part of the membrane neck and widens as the funnel grows away from the bud.
Ladinsky, M. S. et al. Electron tomography of HIV-1 infection in gut-associated lymphoid tissue. PLoS Pathog. 10, e1003899 (2014).
Rozycki, B., Boura, E., Hurley, J. H. & Hummer, G. Membrane-elasticity model of coatless vesicle budding induced by ESCRT complexes. PLoS Comput. Biol. 8, e1002736 (2012).
Mercker, M. & Marciniak-Czochra, A. Bud-neck scaffolding as a possible driving force in ESCRT-induced membrane budding. Biophys. J. 108, 833–843 (2015).
Teis, D., Saksena, S., Judson, B. L. & Emr, S. D. ESCRT-II coordinates the assembly of ESCRT-III filaments for cargo sorting and multivesicular body vesicle formation. EMBO J. 29, 871–883 (2010).
Im, Y. J., Wollert, T., Boura, E. & Hurley, J. H. Structure and function of the ESCRT-II–III interface in multivesicular body biogenesis. Dev. Cell 17, 234–243 (2009).
Strack, B., Calistri, A., Craig, S., Popova, E. & Gottlinger, H. G. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114, 689–699 (2003).
von Schwedler, U. K. et al. The protein network of HIV budding. Cell 114, 701–713 (2003).
Kim, J. et al. Structural basis for endosomal targeting by the Bro1 domain. Dev. Cell 8, 937–947 (2005).
Lee, S., Joshi, A., Nagashima, K., Freed, E. O. & Hurley, J. H. Structural basis for viral late-domain binding to Alix. Nat. Struct. Mol. Biol. 14, 194–199 (2007).
Fisher, R. D. et al. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell 128, 841–852 (2007).
Pires, R. et al. A crescent-shaped ALIX dimer targets ESCRT-III CHMP4 filaments. Structure 17, 843–856 (2009).
McCullough, J., Fisher, R. D., Whitby, F. G., Sundquist, W. I. & Hill, C. P. ALIX-CHMP4 interactions in the human ESCRT pathway. Proc. Natl Acad. Sci. USA 105, 7687–7691 (2008).
Dowlatshahi, D. P. et al. ALIX is a Lys63-specific polyubiquitin binding protein that functions in retrovirus budding. Dev. Cell 23, 1247–1254 (2012).
Keren-Kaplan, T. et al. Structure-based in silico identification of ubiquitin-binding domains provides insights into the ALIX-V:ubiquitin complex and retrovirus budding. EMBO J. 32, 538–551 (2013).
Pashkova, N. et al. The yeast Alix homolog Bro1 functions as a ubiquitin receptor for protein sorting into multivesicular endosomes. Dev. Cell 25, 520–533 (2013).
Zhai, Q. T. et al. Activation of the retroviral budding factor ALIX. J. Virol. 85, 9222–9226 (2011).
Ali, N. et al. Recruitment of UBPY and ESCRT exchange drive HD-PTP-dependent sorting of EGFR to the MVB. Curr. Biol. 23, 453–461 (2013).
Loncle, N., Agromayor, M., Martin-Serrano, J. & Williams, D. W. An ESCRT module is required for neuron pruning. Sci. Rep. 5, 8461 (2015).
Parkinson, M. D. J. et al. A non-canonical ESCRT pathway, including histidine domain phosphotyrosine phosphatase (HD-PTP), is used for down-regulation of virally ubiquitinated MHC class I. Biochem. J. 471, 79–88 (2015).
Muziol, T. et al. Structural basis for budding by the ESCRT-III factor CHMP3. Dev. Cell 10, 821–830 (2006).
Tang, S. et al. Structural basis for activation, assembly and membrane binding of ESCRT-III Snf7 filaments. eLife http://dx.doi.org/10.7554/eLife.12548 (2015).
Bajorek, M. et al. Structural basis for ESCRT-III protein autoinhibition. Nat. Struct. Mol. Biol. 16, 754–762 (2009).
Xiao, J. Y. et al. Structural basis of Ist1 function and Ist1–Did2 interaction in the multivesicular body pathway and cytokinesis. Mol. Biol. Cell 20, 3514–3524 (2009).
McCullough, J. et al. Structure and membrane remodeling activity of ESCRT-III helical polymers. Science 350, 1548–1551 (2015). Reveals that the atomic resolution structure of a CHMP1B–IST1 tube shows marked structural rearrangements in CHMP1B and that this particular combination of ESCRTs carries out normal-topology scission.
Zamborlini, A. et al. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc. Natl Acad. Sci. USA 103, 19140–19145 (2006).
Shim, S., Kimpler, L. A. & Hanson, P. I. Structure/function analysis of four core ESCRT-III proteins reveals common regulatory role for extreme C-terminal domain. Traffic 8, 1068–1079 (2007).
Lata, S. et al. Structural basis for autoinhibition of ESCRT-III CHMP3. J. Mol. Biol. 378, 818–827 (2008).
Henne, W. M., Buchkovich, N. J., Zhao, Y. & Emr, S. D. The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices. Cell 151, 356–371 (2012).
Róz˙ycki, B., Kim, Y. C. & Hummer, G. SAXS ensemble refinement of ESCRT-III CHMP3 conformational transitions. Structure 19, 109–116 (2011).
Schuh, A. L. et al. The VPS-20 subunit of the endosomal sorting complex ESCRT-III exhibits an open conformation in the absence of upstream activation. Biochem. J. 466, 625–637 (2015).
Hanson, P. I., Roth, R., Lin, Y. & Heuser, J. E. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J. Cell Biol. 180, 389–402 (2008).
Shen, Q.-T. et al. Structural analysis and modeling reveals new mechanisms governing ESCRT-III spiral filament assembly. J. Cell Biol. 206, 763–777 (2014).
Chiaruttini, N. et al. Relaxation of loaded ESCRT-III spiral springs drives membrane deformation. Cell 163, 866–879 (2015). Shows that the mechanical properties of ESCRTs are consistent with the action of these proteins as spiral springs.
Lata, S. et al. Helical structures of ESCRT-III are disassembled by VPS4. Science 321, 1354–1357 (2008).
Effantin, G. et al. ESCRT-III CHMP2A and CHMP3 form variable helical polymers in vitro and act synergistically during HIV-1 budding. Cell. Microbiol. 15, 213–226 (2013).
Bodon, G. et al. Charged multivesicular body protein 2B (CHMP2B) of the endosomal sorting complex required for transport-III (ESCRT-III) polymerizes into helical structures deforming the plasma membrane. J. Biol. Chem. 286, 40276–40286 (2011).
Allison, R. et al. An ESCRT–spastin interaction promotes fission of recycling tubules from the endosome. J. Cell Biol. 202, 527–543 (2013).
Dobro, M. J. et al. Electron cryotomography of ESCRT assemblies and dividing Sulfolobus cells suggests that spiraling filaments are involved in membrane scission. Mol. Biol. Cell 24, 2319–2327 (2013).
Guizetti, J. et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 331, 1616–1620 (2011).
Baumgartel, V. B. V. et al. Live-cell visualization of dynamics of HIV budding site interactions with an ESCRT component. Nat. Cell Biol. 13, 469–474 (2011).
Jouvenet, N. J. N., Zhadina, M., Bieniasz, P. D. & Simon, S. M. Dynamics of ESCRT protein recruitment during retroviral assembly. Nat. Cell Biol. 13, 394–401 (2011).
Carlton, J. G. & Martin-Serrano, J. Parallels between cytokinesis and retroviral budding: A role for the ESCRT machinery. Science 316, 1908–1912 (2007).
Agromayor, M. & Martin-Serrano, J. Knowing when to cut and run: Mechanisms that control cytokinetic abscission. Trends Cell Biol. 23, 433–441 (2013).
Elia, N., Sougrat, R., Spurlin, T., Hurley, J. H. & Lippincott-Schwartz, J. Dynamics of ESCRT machinery during cytokinesis and its role in abscission. Proc. Natl Acad. Sci. USA 108, 4846–4851 (2011).
Lee, I. H., Kai, H., Carlson, L. A., Groves, J. T. & Hurley, J. H. Negative membrane curvature catalyzes nucleation of endosomal sorting complex required for transport (ESCRT)-III assembly. Proc. Natl Acad. Sci. USA 112, 15892–15897 (2015). Uses real-time and super-resolution imaging of ESCRT-III polymerization on nanofabricated concave templates to show that ESCRT-III nucleation has a negative curvature preference.
Teis, D., Saksena, S. & Emr, S. D. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev. Cell 15, 578–589 (2008).
Babst, M., Wendland, B., Estepa, E. J. & Emr, S. D. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17, 2982–2993 (1998).
Monroe, N. & Hill, C. P. Meiotic clade AAA ATPases: protein polymer disassembly machines. J. Mol. Biol. 428, 1897–1911 (2016).
Hurley, J. H. & Yang, D. MIT domainia. Dev. Cell 14, 6–8 (2008).
Monroe, N. et al. The oligomeric state of the active Vps4 AAA ATPase. J. Mol. Biol. 426, 510–525 (2013).
Caillat, C. et al. Asymmetric ring structure of Vps4 required for ESCRT-III disassembly. Nat. Commun. 6, 8781 (2015). Provides the first structure of the active hexameric form of Vps4.
Obita, T. et al. Structural basis for selective recognition of ESCRT-III by the AAA ATPase Vps4. Nature 449, 735–739 (2007).
Stuchell-Brereton, M. et al. ESCRT-III recognition by VPS4 ATPases. Nature 449, 740–744 (2007).
Guo, E. Z. & Xu, Z. Distinct mechanisms of recognizing endosomal sorting complex required for transport III (ESCRT-III) protein IST1 by different microtubule interacting and trafficking (MIT) domains. J. Biol. Chem. 290, 8396–8408 (2015).
Kieffer, C. et al. Two distinct modes of ESCRT-III recognition are required for VPS4 functions in lysosomal protein targeting and HIV-1 budding. Dev. Cell 15, 62–73 (2008).
Shim, S., Merrill, S. A. & Hanson, P. I. Novel interactions of ESCRT-III with LIP5 and VPS4 and their implications for ESCRT-III disassembly. Mol. Biol. Cell 19, 2661–2672 (2008).
Xiao, J. et al. Structural basis of Vta1 function in the multi-vesicular body sorting pathway. Dev. Cell 14, 37–49 (2008).
Solomons, J. et al. Structural basis for ESCRT-III CHMP3 recruitment of AMSH. Structure 19, 1149–1159 (2011).
Merrill, S. A. & Hanson, P. I. Activation of human VPS4A by ESCRT-III proteins reveals ability of substrates to relieve enzyme autoinhibition. J. Biol. Chem. 285, 35428–35438 (2010).
Norgan, A. P. et al. Relief of autoinhibition enhances Vta1 activation of Vps4 via the Vps4 stimulatory element. J. Biol. Chem. 288, 26147–26156 (2013).
Davies, B. A. et al. Vps4 stimulatory element of the cofactor Vta1 contacts the ATPase Vps4 α7 and α9 to stimulate ATP hydrolysis. J. Biol. Chem. 289, 28707–28718 (2014).
Han, H. et al. Binding of substrates to the central pore of the Vps4 ATPase is autoinhibited by the microtubule interacting and trafficking (MIT) domain and activated by MIT interacting motifs (MIMs). J. Biol. Chem. 290, 13490–13499 (2015).
Scott, A. et al. Structural and mechanistic studies of VPS4 proteins. EMBO J. 24, 3658–3669 (2005).
Gonciarz, M. D. et al. Biochemical and structural studies of yeast Vps4 oligomerization. J. Mol. Biol. 384, 878–895 (2008).
Hartmann, C. et al. Vacuolar protein sorting: Two different functional states of the AAA-ATPase Vps4p. J. Mol. Biol. 377, 352–363 (2008).
Yu, Z. H., Gonciarz, M. D., Sundquist, W. I., Hill, C. P. & Jensen, G. J. Cryo-EM structure of dodecameric Vps4p and its 2:1 complex with Vta1p. J. Mol. Biol. 377, 364–377 (2008).
Landsberg, M. J., Vajjhala, P. R., Rothnagel, R., Munn, A. L. & Hankamer, B. Three-dimensional structure of AAA ATPase Vps4: Advancing structural insights into the mechanisms of endosomal sorting and enveloped virus budding. Structure 17, 427–437 (2009).
Yang, B., Stjepanovic, G., Shen, Q. T., Martin, A. & Hurley, J. H. Vps4 disassembles an ESCRT-III filament by global unfolding and processive translocation. Nat. Struct. Mol. Biol. 22, 492–498 (2015). Shows that Vps4 disassembles ESCRT-III by unfolding the subunits and pulling them through the central pore.
Azmi, I. F. et al. ESCRT-III family members stimulate Vps4 ATPase activity directly or via Vta1. Dev. Cell 14, 50–61 (2008).
Yang, D. & Hurley, J. H. Structural role of the Vps4-Vta1 interface in ESCRT-III recycling. Structure 18, 976–984 (2010).
Fabrikant, G. et al. Computational model of membrane fission catalyzed by ESCRT-III. PLoS Comput. Biol. 5, e1000575 (2009).
Wollert, T., Wunder, C., Lippincott-Schwartz, J. & Hurley, J. H. Membrane scission by the ESCRT-III complex. Nature 458, 172–177 (2009).
Van Engelenburg, S. B. et al. Distribution of ESCRT machinery at HIV assembly sites reveals virus scaffolding of ESCRT subunits. Science 343, 653–656 (2014).
Prescher, J. et al. Super-resolution imaging of ESCRT-proteins at HIV-1 assembly sites. PLoS Pathog. 11, e1004677 (2015).
Henne, W. M., Buchkovich, N. J. & Emr, S. D. The ESCRT pathway. Dev. Cell 21, 77–91 (2011).
Hurley, J. H. & Hanson, P. I. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat. Rev. Mol. Cell Biol. 11, 556–566 (2010).
McCullough, J., Colf, L. A. & Sundquist, W. I. Membrane fission reactions of the mammalian ESCRT pathway. Annu. Rev. Biochem. 82, 663–692 (2013).
Lenz, M., Crow, D. J. G. & Joanny, J. F. Membrane buckling induced by curved filaments. Phys. Rev. Lett. 103, 038101 (2009).
Carlson, L.-A., Shen, Q.-T., Pavlin, M. R. & Hurley, J. H. ESCRT filaments as spiral springs. Dev. Cell 35, 397–398 (2015).
Schekman, R. & Orci, L. Coat proteins and vesicle budding. Science 271, 1526–1533 (1996).
Bonifacino, J. S. & Glick, B. S. The mechanisms of vesicle budding and fusion. Cell 116, 153–166 (2004).
Nickerson, D. P., Russell, D. W. & Odorizzi, G. A concentric circle model of multivesicular body cargo sorting. EMBO Rep. 8, 644–650 (2007).
Odorizzi, G. Membrane manipulations by the ESCRT machinery. F1000Res. 4, 516 (2015).
Vietri, M. et al. Spastin and ESCRT-III coordinates mitotic spindle disassembly and nuclear envelope resealing. Nature 522, 231–235 (2015).
Zhai, Q. et al. Structural and functional studies of ALIX interactions with YPXnL late domains of HIV-1 and EIAV. Nat. Struct. Mol. Biol. 15, 43–49 (2008).
Bauer, I., Brune, T., Preiss, R. & Kölling, R. Evidence for a nonendosomal function of the Saccharomyces cerevisiae ESCRT-III-like protein Chm7. Genetics 201, 1439–1452 (2015).
Acknowledgements
Research on endosomal sorting complexes required for transport (ESCRTs) in the Hurley laboratory is supported by the US National Institutes of Health, grant AI112442. J.H.I. is supported by the Center for the Structural Biology of Cellular Host Elements in Egress, Trafficking, and Assembly of HIV (CHEETAH), US National Institutes of Health, grant GM082545.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
ESCRT subunit nomenclature (PDF 77 kb)
Supplementary information S2 (movie)
Animation of the dome model of ESCRT-mediated reverse-topology membrane scission. See legend to Fig. 7a for explanation of features. ESCRT-I (green), ESCRT-II (orange), ESCRT-III (yellow), ALIX (purple), VPS4 (pink), Gag (blue). (MOV 50707 kb)
Supplementary information S3 (movie)
Animation of the reverse dome model of ESCRT-mediated reverse-topology membrane scission. See Fig. 7b. ESCRT-I (green), ESCRT-II (orange), ESCRT-III (yellow), ALIX (purple), VPS4 (pink), Gag (blue). (MOV 51580 kb)
Supplementary information S4 (movie)
Animation of the buckling model of ESCRT-mediated reverse-topology membrane scission. See Fig. 7c. ESCRT-I (green), ESCRT-II (orange), ESCRT-III (yellow), ALIX (purple), VPS4 (pink), Gag (blue). (MOV 52453 kb)
Supplementary information S5 (movie)
Animation of the possible role of ESCRT proteins in endocytic cargo sorting. Cargo proteins (red) recruit ESCRT-I (green) and ESCRT-II (orange). ESCRT-III (yellow) is recruited by ESCRT-II and grows in concentric rings of increasing diameter, forming a flat disc. The addition of a second type of ESCRT-III protein (teal) causes buckling of the ESCRT-III disc, forming a cone. Depolymerization of second ESCRT-III rings by VPS4 (pink) allows ESCRT-III to return to a flattened disc morphology and for membrane fission to occur. (MOV 117858 kb)
Glossary
- Membrane necks
-
Narrow membranous connections linking two entities, including: nascent endosomes, exosomes, enveloped viruses and intraluminal vesicles to their membranes of origin; daughter cell to mother cell or another daughter cell; and the cytosol to the lumen of a double-membrane structure, such as the nucleus or a nascent autophagosome.
- Reverse-topology scission
-
The severing of membrane necks when the scission factors function from the membrane face contiguous with the interior of the neck.
- Multivesicular bodies
-
(MVBs). Late endosomes containing internal vesicles.
- Cytokinesis
-
The separation of daughter cells, which is the final stage in cell division, in which the membrane and microtubules connecting the two cells are severed.
- Ubiquitin
-
A 76-amino-acid protein whose covalent conjugation to target proteins can (among many other fates) mark them as substrates for the endosomal sorting complex required for transport (ESCRT) system.
- Giant unilamellar vesicles
-
Synthetic vesicles of ∼5–50 μm in diameter; a popular model system for in vitro studies of membrane remodelling.
- Nucleation
-
The kinetic step in which an initial seed unit is assembled that, when formed, can readily grow into a larger structure.
- Intercellular bridge
-
The narrow membranous and microtubule-containing structure connecting two daughter cells immediately before their separation in cytokinesis.
Rights and permissions
About this article
Cite this article
Schöneberg, J., Lee, IH., Iwasa, J. et al. Reverse-topology membrane scission by the ESCRT proteins. Nat Rev Mol Cell Biol 18, 5–17 (2017). https://doi.org/10.1038/nrm.2016.121
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2016.121
This article is cited by
-
A heterozygous germline deletion within USP8 causes severe neurodevelopmental delay with multiorgan abnormalities
Journal of Human Genetics (2024)
-
The role and applications of extracellular vesicles in osteoporosis
Bone Research (2024)
-
Brominated lipid probes expose structural asymmetries in constricted membranes
Nature Structural & Molecular Biology (2023)
-
Cell entry and release of quasi-enveloped human hepatitis viruses
Nature Reviews Microbiology (2023)
-
The plant unique ESCRT component FREE1 regulates autophagosome closure
Nature Communications (2023)