Key Points
-
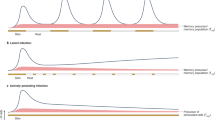
CD8+ T-cell memory to viruses is stable, but memory is lost after infections with other viruses.
-
The homeostasis of CD8+ T-cell memory differs from that of CD4+ T-cell memory.
-
Crossreactive T-cell responses between heterologous viruses might be a common event.
-
The immunodominance of epitopes that are recognized by T cells is, in part, a function of a T-cell repertoire that is moulded by the past history of infection.
-
Viruses might cause the activation of memory T cells that are specific for previously encountered pathogens.
-
Memory T cells that are specific for unrelated pathogens might have roles in protective immunity and immunopathology caused by heterologous infectious agents.
-
Immune deviation, or the balance between T helper 1 (TH1) and TH2 responses, might be influenced by the memory T-cell pool that is specific for previously encountered pathogens.
Abstract
Memory T cells that are specific for one virus can become activated during infection with an unrelated heterologous virus, and might have roles in protective immunity and immunopathology. The course of each infection is influenced by the T-cell memory pool that has been laid down by a host's history of previous infections, and with each successive infection, T-cell memory to previously encountered agents is modified. Here, we discuss evidence from studies in mice and humans that shows the importance of this phenomenon in determining the outcome of infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Matthew, A. et al. Predominance of HLA-restricted cytotoxic T-lymphocyte responses to serotype-cross-reactive epitopes on nonstructural proteins following natural secondary dengue virus infection. J. Virol. 72, 3999–4004 (1998).This study shows that different dengue-virus serotypes have high homology in terms of T-cell epitopes, and they induce crossreactive T-cell responses.
Halstead, S. B. Antibody, macrophages, dengue-virus infection, shock and hemorrhage: a pathogenetic cascade. Rev. Infect. Dis. 11, S830–S839 (1989).
Bjorkman, P. J. MHC restriction in three dimensions: a view of T-cell receptor/ligand interactions. Cell 89, 167–170 (1997).
Yewdell, J. W. & Bennink, J. R. Immunodominance in major histocompatibility complex class-I-restricted T-lymphocyte responses. Annu. Rev. Immunol. 17, 51–88 (1999).
Falk, K., Rotzschke, O., Stevanovic, S., Jung, G. & Rammensee, H. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351, 290–296 (1991).
Kaech, S. M. & Ahmed, R. Memory CD8+ T-cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nature Immunol. 2, 415–422 (2001).
van Stipdonk, M. J., Lemmens, E. E. & Schoenberger, S. P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nature Immunol. 2, 423–429 (2001).
Mercado, R. et al. Early programming of T-cell populations responding to bacterial infection. J. Immunol. 165, 6833–6839 (2000).
Selin, L. K., Vergilis, K., Welsh, R. M. & Nahill, S. R. Reduction of otherwise remarkably stable virus-specific cytotoxic T-lymphocyte memory by heterologous viral infections. J. Exp. Med. 183, 2489–2499 (1996).This study quantifies the expanding number of virus-specific CD8+ T cells during viral infections, and shows that this population remains stable in long-term memory, and that heterologous virus infections disrupt this stability and cause reductions in the memory response to previously encountered viruses.
Razvi, E. S., Jiang, Z., Woda, B. A. & Welsh, R. M. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr and Bcl-2-transgenic mice. Am. J. Pathol. 147, 79–91 (1995).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).Memory T cells reside at high frequencies in peripheral organs.
Marshall, D. R. et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl Acad. Sci. USA 98, 6313–6318 (2001).This report describes how memory CD8+ T cells migrate into peripheral organs as they disappear from the lymphoid organs at the end stage of the T-cell response to viral infections.
Van der Most, R. G. et al. Identification of Db- and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology 240, 158–167 (1998).
Chen, W., Anton, L. C., Bennink, J. R. & Yewdell, J. W. Dissecting the multifactorial causes of immunodominance in class-I-restricted T-cell responses to viruses. Immunity 12, 83–93 (2000).
Vitiello, A. et al. Immunodominance analysis of CTL responses to influenza PR8 virus reveals two dominant and subdominant Kb-restricted epitopes. J. Immunol. 157, 5555–5562 (1996).
Stevenson, P. G., Belz, G. T., Altman, J. D. & Doherty, P. C. Changing patterns of dominance in the CD8+ T-cell response during acute and persistent murine γ-herpesvirus infection. Eur. J. Immunol. 29, 1059–1067 (1999).
Wallace, M. E., Keating, R., Heath, W. R. & Carbone, F. R. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J. Virol. 73, 7619–7626 (1999).
Belz, G. T., Stevenson, P. G. & Doherty, P. C. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T-cell response to influenza A viruses. J. Immunol. 165, 2404–2409 (2000).
Lin, M. Y. & Welsh, R. M. Stability and diversity of T-cell receptor (TCR) repertoire usage during lymphocytic choriomeningitis virus infection of mice. J. Exp. Med. 188, 1993–2005 (1998).The virus-induced T-cell repertoire usage differs between genetically identical mice, even though the specificity of the CD8+ T-cell response is similar.
Bousso, P. et al. Individual variations in the murine T-cell response to a specific peptide reflect variability in naive repertoire. Immunity 9, 169–178 (1998).
Blattman, J. N., Sourdive, D. J., Murali-Krishna, K., Ahmed, R. & Altman, J. D. Evolution of the T-cell repertoire during primary, memory and recall responses to viral infection. J. Immunol. 165, 6081–6090 (2000).
Mason, D. A very high level of crossreactivity is an essential feature of the T-cell repertoire. Immunol. Today 19, 395–404 (1998).This paper provides theoretical calculations that indicate that T cells must be highly crossreactive.
Tabi, Z., Lynch, F., Ceredig, R., Allan, J. E. & Doherty, P. C. Virus-specific memory T cells are Pgp-1+ and can be selectively activated with phorbol ester and calcium ionophore. Cell. Immunol. 113, 268–277 (1988).
Bradley, L. M., Croft, M. & Swain, S. L. T-cell memory: new perspectives. Immunol. Today 14, 197–199 (1993).
Pihlgren, M., Dubois, P. M., Tomkowiak, M., Sjogren, T. & Marvel, J. Resting memory CD8+ T cells are hyperactive to antigenic challenge in vitro. J. Exp. Med. 184, 2141–2151 (1996).
Curtsinger, J. M., Lins, D. C. & Mescher, M. F. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells (CD44low, Ly6C−) to TCR/CD8 signaling in response to antigen. J. Immunol. 160, 3236–3243 (1998).
Veiga-Fernandes, H., Walter, U., Bourgeois, C., McLean, A. & Rocha, B. Response of naive and memory CD8 T cells to antigen stimulation in vivo. Nature Immunol. 1, 47–53 (2000).
Sheil, J. M., Bevan, M. J. & Lefrancois, L. Characterization of dual-reactive H-2Kb-restricted anti-vesicular stomatitis virus and alloreactive cytotoxic T cells. J. Immunol. 138, 3654–3660 (1987).
Braciale, T. J., Andrew, M. E. & Braciale, V. L. Simultaneous expression of H-2-restricted and alloreactive recognition by a cloned line of influenza virus-specific cytotoxic T lymphocytes. J. Exp. Med. 153, 1371–1376 (1981).
Anderson, R. W., Bennick, J. R., Yewdell, J. W., Maloy, W. L. & Coligan, J. E. Influenza basic polymerase 2 peptides are recognized by influenza nucleoprotein-specific cytotoxic T lymphocytes. Mol. Immunol. 29, 1089–1096 (1992).
Kuwano, K., Reyes, R. E., Humphreys, R. E. & Ennis, F. A. Recognition of disparate HA and NS1 peptides by an H-2kd-restricted, influenza-specific CTL clone. Mol. Immunol. 28, 1–7 (1991).
Yang, H. & Welsh, R. M. Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J. Immunol. 136, 1186–1193 (1986).
Tomkinson, B. E., Maziarz, R. & Sullivan, J. L. Characterization of the T-cell-mediated cellular cytotoxicity during infectious mononucleosis. J. Immunol. 143, 660–670 (1989).
Strang, G. & Rickinson, A. B. Multiple HLA class-I-dependent cytotoxicities constitute the 'non-HLA-restricted' response in infectious mononucleosis. Eur. J. Immunol. 17, 1007–1013 (1987).
Burrows, S. R. et al. Cross-reactive memory T cells for Epstein–Barr virus augment the alloresponse to common human leukocyte antigens: degenerate recognition of major histocompatibility complex-bound peptide by T cells and its role in alloreactivity. Eur. J. Immunol. 27, 1726–1736 (1997).
Burrows, S. R., Khanna, R., Silins, S. L. & Moss, D. J. The influence of antiviral T-cell responses on the alloreactive repertoire. Immunol. Today 20, 203–207 (1999).
Nahill, S. R. & Welsh, R. M. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T-lymphocyte response. J. Exp. Med. 177, 317–327 (1993).
Selin, L. K., Nahill, S. R. & Welsh, R. M. Cross-reactivities in memory cytotoxic T-lymphocyte recognition of heterologous viruses. J. Exp. Med. 179, 1933–1943 (1994).
Yang, H., Dundon, P. L., Nahill, S. R. & Welsh, R. M. Virus-induced polyclonal cytotoxic T-lymphocyte stimulation. J. Immunol. 142, 1710–1718 (1989).
Daniel, C., Horvath, S. & Allen, P. M. A basis for alloreactivity: MHC helical residues broaden peptide recognition by the TCR. Immunity 8, 543–552 (1998).
Speir, J. A. et al. Structural basis of 2C TCR allorecognition of H-2Ld peptide complexes. Immunity 8, 553–562 (1998).
Alam, S. M. & Gascoigne, N. R. Posttranslational regulation of TCR Vα allelic exclusion during T-cell differentiation. J. Immunol. 160, 3883–3890 (1998).
Welsh, R. M. et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J. Virol. 74, 2210–2218 (2000).
Betts, M. R. et al. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74, 9144–9151 (2000).These authors show that predictable hierarchies of immunodominant epitopes of HIV are not seen in the 'wild' human population.
Day, C. L. et al. Relative dominance of epitope-specific cytotoxic T-lymphocyte responses in human immunodeficiency virus type-1-infected persons with shared HLA alleles. J. Virol. 75, 6279–6291 (2001).
Fazekas de St Groth, S. & Webster, R. G. Disquisitions on original antigenic sin. II. Proof in lower creatures. J. Exp. Med. 124, 347–361 (1966).
Haanan, J. B., Wolkers, M. C., Kruisbeek, A. M. & Schumacher, T. N. Selective expansion of cross-reactive CD8+ memory T cells by viral variants. J. Exp. Med. 190, 1319–1328 (1999).This study used viral strain-specific tetramers to show that a related virus will selectively stimulate the expansion of crossreactive but not non-crossreactive CD8+ T-cell populations during infection.
Klenerman, P. & Zinkernagel, R. M. Original antigenic sin impairs cytotoxic T-lymphocyte responses to viruses bearing variant epitopes. Nature 394, 421–422 (1998).
Tough, D. F., Borrow, P. & Sprent, J. Induction of bystander T-cell proliferation by viruses and type I interferon in vivo. Science 272, 1947–1950 (1996).
Sprent, J., Zhang, X., Sun, S. & Tough, D. T-cell turnover in vivo and the role of cytokines. Immunol. Lett. 65, 21–25 (1999).
Zarozinski, C. C. & Welsh, R. M. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T-cell response. J. Exp. Med. 185, 1629–1639 (1997).
McNally, J. M. et al. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J. Virol. 75, 5965–5976 (2001).This report shows that non-virus-specific 'bystander' CD8+ T cells are reduced in number during virus infections and that type I IFN induces the apoptosis of memory CD8+ T cells.
Mahalingam, S., Foster, P. S., Lobigs, S., Farber, J. M. & Karupiah, G. Interferon-inducible chemokines and immunity to poxvirus infections. Immunol. Rev. 177, 127–133 (2000).
Topham, D. J., Castrucci, M., Wingo, F. S., Belz, G. T. & Doherty, P. C. The role of antigen in the localization of naive, acutely activated and memory CD8+ T cells to the lung during influenza pneumonia. J. Immunol. 167, 6983–6990 (2001).
Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678 (2000).
Flynn, K. J., Riberdy, J. M., Christensen, J. P., Altman, J. D. & Doherty, P. C. In vivo proliferation of naive and memory influenza-specific CD8+ T cells. Proc. Natl Acad. Sci. USA 96, 8597–8602 (1999).
Belz, G. T. & Doherty, P. C. Virus-specific and bystander CD8+ T-cell proliferation in the persistent phases of a γ-herpesvirus infection. J. Virol. 75, 4435–4438 (2001).
Turner, S. J., Cross, R., Xie, W. & Doherty, P. C. Concurrent naive and memory CD8+ T-cell responses to an influenza virus. J. Immunol. 167, 2753–2758 (2001).
Lau, L. L., Jamieson, B. D., Somasundaram, T. & Ahmed, R. Cytotoxic T-cell memory without antigen. Nature 369, 648–652 (1994).
Homann, D., Teyton, L. & Oldstone, M. B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ memory. Nature Med. 7, 892–893 (2001).
Razvi, E. S., Welsh, R. M. & McFarland, H. I. In vivo state of antiviral CTL precursors: characterization of a cycling population containing CTL precursors in immune mice. J. Immunol. 154, 620–632 (1995).
Sprent, J. & Tough, D. F. Lymphocyte life-span and memory. Science 265, 1395–1400 (1994).
Zimmermann, C., Brduscha-Riem, K., Blaser, C., Zinkernagel, R. M. & Pircher, H. Visualization, characterization and turnover of CD8+ memory T cells in virus-infected hosts. J. Exp. Med. 183, 1367–1375 (1996).
Selin, L. K. et al. Attrition of T-cell memory: selective loss of lymphocytic choriomeningitis virus (LCMV) epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11, 733–742 (1999).This study shows that CD8+ T cells that are specific for previously encountered viruses are reduced in number by heterologous viral infections, and there is a selective loss of some specificities but not others.
Chen, H. D. et al. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nature Immunol. 2, 1067–1076 (2001).This study shows the recruitment and activation of LCMV-specific memory T cells into the lung during vaccinia virus infection, which results in marked immunopathology in a respiratory model of heterologous immunity.
Varga, S. M. & Welsh, R. M. Cutting edge: detection of a high frequency of virus-specific CD4+ T cells during acute infection with lymphocytic choriomeningitis virus. J. Immunol. 161, 3215–3218 (1998).
Varga, S. M. & Welsh, R. M. High frequency of virus-specific interleukin-2-producing CD4+ T cells and TH1 dominance during lymphocytic choriomeningitis virus infection. J. Virol. 74, 4429–4432 (2000).
Varga, S. M., Selin, L. K. & Welsh, R. M. Independent regulation of lymphocytic choriomeningitis virus-specific T-cell memory pools: relative stability of CD4 memory under conditions of CD8 memory T-cell loss. J. Immunol. 166, 1554–1561 (2001).This study shows that heterologous viral infections cause less of a decline in CD4+ T-cell memory than they do in CD8+ T-cell memory.
Selin, L. K., Varga, S. M., Wong, I. C. & Welsh, R. M. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T-cell populations. J. Exp. Med. 188, 1705–1715 (1998).This shows the principle of heterologous immunity and immunopathology during viral infections.
Schlesinger, C., Meyer, C. A., Veeraraghavan, S. & Koss, M. N. Constrictive (obliterative) bronchiolitis: diagnosis, etiology and a critical review of the literature. Ann. Diagn. Pathol. 2, 321–334 (1998).
Ploegh, H. L. Viral strategies of immune evasion. Science 280, 248–253 (1998).
Aaby, P. et al. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ 311, 481–485 (1995).
Doherty, P. C. et al. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159, 105–117 (1997).
Jameson, J., Cruz, J. & Ennis, F. A. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J. Virol. 72, 8682–8689 (1998).This paper identifies several influenza virus T-cell epitopes, some of which are crossreactive between strains.
Yang, H., Joris, I., Majno, G. & Welsh, R. M. Necrosis of adipose tissue induced by sequential infections with unrelated viruses. Am. J. Pathol. 120, 173–177 (1985).
Bolognia, J. & Braverman, I. M. In Harrison's Principles of Internal Medicine (eds Isselbacher, K. J. et al.) 290–307 (McGraw–Hill, New York, 1992).
Zhao, Z.-S., Granucci, F., Yeh, L., Schaffer, P. A. & Cantor, H. Molecular mimicry by herpes simplex virus type-1: autoimmune disease after viral infection. Science 279, 1344–1347 (1998).
Evans, C. F., Horwitz, M. S., Hobbs, M. V. & Oldstone, M. B. Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J. Exp. Med. 184, 2371–2384 (1996).This study shows that a virus can break tolerance to a transgene in the brain and induce transient encephalitis, which will undergo remission until exacerbated by a heterologous virus infection.
Swain, S. L. Helper T-cell differentiation. Curr. Opin. Immunol. 11, 180–185 (1999).
Ismail, N. & Bretscher, P. A. More antigen-dependent CD4+ T cell/CD4+ T cell interactions are required for the primary generation of TH2 than of TH1 cells. Eur. J. Immunol. 31, 1765–1771 (2001).
Swain, S. L. Interleukin-18: tipping the balance towards a T helper cell 1 response. J. Exp. Med. 194, F11–F14 (2001).
Cohn, L., Herrick, C., Niu, N., Homer, R. & Bottomly, K. IL-4 promotes airways eosinophilia by suppressing IFN-γ production: defining a novel role for IFN-γ in the regulation of allergic airway inflammation. J. Immunol. 166, 2760–2767 (2001).
Rook, G. A. & Stanford, J. L. Give us this day our daily germs. Immunol. Today 19, 113–116 (1998).
Varga, S. M., Wang, X., Welsh, R. M. & Braciale, T. J. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4+ T cells. Immunity 15, 637–646 (2001).
Kapikian, A. Z., Mitchell, R. H., Chanock, R. M., Shvedoff, R. A. & Stewart, C. E. An epidemiological study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 89, 405–421 (1969).
Cohn, L., Homer, R. J., Niu, N. & Bottomly, K. T helper 1 cells and interferon-γ regulate allergic airway inflammation and mucus production. J. Exp. Med. 190, 1309–1318 (1999).
Graham, B. S., Bunton, L. A., Wright, P. F. & Karzon, D. T. Role of T-lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 88, 1026–1033 (1991).
Walzl, G., Tafuro, S., Moss, P., Openshaw, P. J. & Hussell, T. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J. Exp. Med. 192, 1317–1326 (2000).A heterologous influenza-virus infection can alter the ability of a vaccinia-virus recombinant to prime a host to make a damaging T H 2-like response to RSV.
Johnson, T. R. & Graham, B. S. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13 and eosinophilia by an IL-4-dependent mechanism. J. Virol. 73, 8485–8495 (1999).
Shirakawa, T., Enomoto, T., Shimazu, S. & Hopkin, J. M. The inverse association between tuberculin responses and atopic disorder. Science 275, 77–79 (1997).
Martinez, F. D. et al. Asthma and wheezing in the first six years of life. N. Engl. J. Med. 332, 133–138 (1995).
Shaheen, S. O. et al. Measles and atopy in Guinea–Bissau. Lancet 347, 1792–1796 (1996).
Erb, K. J., Holloway, J. W., Sobeck, A., Moll, H. & Le Gros, G. Infection of mice with Mycobacterium bovis bacillus Calmette–Guerin (BCG) suppresses allergen-induced airway eosinophilia. J. Exp. Med. 187, 561–569 (1998).This study shows that a history of BCG infection can render a host refractory to the induction of a T H 2 response by an allergen.
Wedemeyer, H., Mizukoshi, E., Davis, A. R., Bennink, J. R. & Rehermann, B. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J. Virol. 75, 11392–11400 (2001).Defines a strong crossreactive epitope between hepatitis C virus and influenza virus.
Weinstein, L. & Meade, R. H. Respiratory manifestations of chickenpox. Arch. Intern. Med. 98, 91–99 (1956).
Rickinson, A. B. & Kieff, E. In Virology Vol. 2 (eds Fields, B. N. et al.) 2397–2446 (Lippincott–Raven, Philadelphia, 1996).
Moss, D. J., Burrows, S. R., Silins, S. L., Misko, I. & Khanna, R. The immunology of Epstein–Barr virus infection. Philos Trans R Soc Lond B Biol Sci 356, 475–488 (2001).
Kaul, R. et al. CD8+ lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J. Clin. Invest. 107, 1303–1310 (2001).This study provides evidence of HIV-specific T cells in seronegative and HIV-negative subjects at high risk of HIV infection.
Tillmann, H. L. et al. Infection with GB virus C and reduced mortality among HIV-infected patients. N. Engl. J. Med. 345, 715–724 (2001).
Xiang, J. et al. Effect of coinfection with GB virus C on survival among patients with HIV infection. N. Engl. J. Med. 345, 707–714 (2001).
Barnett, L. A. & Fujinami, R. S. Molecular mimicry: a mechanism for autoimmune injury. FASEB J. 6, 840–844 (1992).
Janeway, C. A. Innate immunity acknowledged. Immunologist 3, 198–200 (1995).
Smoller, B. R., Weishar, M. & Gray, M. H. An unusual cutaneous manifestation in Crohn's disease. Arch Pathol Lab Med 114, 609–610 (1990).
Brehm, M. B. et al. T-cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nature Immunol. (in the press). This study shows that cross-reactive CD8+ T-cell responses during heterologous virus infections influence immunodominance, as the T cells that are specific for the cross-reactive memory epitopes dominate acute responses to the second virus and are preferentially maintained in memory of the first virus, whereas non-crossreactive memory T cells are lost.
Acknowledgements
R.M.W. and L.K.S. are supported by the United States National Institutes of Health. The contents of this article are solely the responsibility of the authors and do not represent the official views of the NIH. We thank M. Brehm, A. Fraire, I. Joris, B. Smoller and H. Chen for their collaborations and helpful comments.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Entrez
LocusLink
OMIM
Glossary
- T-HELPER TYPE 1/2
-
(TH1/TH2). At least two distinct subsets of activated CD4+ T cells have been described. TH1 cells produce IFN-γ, lymphotoxin and TNF, and support cell-mediated immunity. TH2 cells produce IL-4, IL-5 and IL-13, support humoral immunity, and downregulate TH1 responses.
- CO-STIMULATION
-
Optimal signalling through the TCR complex requires accessory cell-surface molecules, such as CD28 or LFA1. Signals that are delivered from these molecules contribute to enhancing the immune response. In the absence of these co-stimulatory signals, naive T cells become unresponsive to a subsequent challenge with antigen.
- CFSE
-
(5,6-carboxy-fluorescein diacetate succinimidyl ester). This a fluorescent dye that is used to label cells. With each cell division, the label is distributed equally into daughter cells. The loss of fluorescence intensity is used to calculate the number of cell divisions.
- BYSTANDER ACTIVATION
-
The term, as it is used here, refers to the activation of T cells in which the TCRs are not being triggered by the antigens that are driving the immune response. This activation might be mediated by cytokines.
- CLONAL IMPRINTING/ORIGINAL ANTIGENIC SIN
-
Previous exposure to one virus strain diverts the antibody response after exposure to a second virus strain to epitopes that are shared between the two strains.
- BROMODEOXYURIDINE
-
(BrdU). A thymidine analogue that can be incorporated into DNA during S-phase when cells are exposed to this substance. Cells that have incorporated BrdU, and presumably have divided, can be visualized with anti-BrdU antibodies using flow cytometry.
Rights and permissions
About this article
Cite this article
Welsh, R., Selin, L. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol 2, 417–426 (2002). https://doi.org/10.1038/nri820
Issue Date:
DOI: https://doi.org/10.1038/nri820
This article is cited by
-
Understanding immunity: an alternative framework beyond defense and strength
Biology & Philosophy (2023)
-
Significance of bystander T cell activation in microbial infection
Nature Immunology (2022)
-
The double-sided effects of Mycobacterium Bovis bacillus Calmette–Guérin vaccine
npj Vaccines (2021)
-
The dichotomous and incomplete adaptive immunity in COVID-19 patients with different disease severity
Signal Transduction and Targeted Therapy (2021)
-
Aging increases the systemic molecular degree of inflammatory perturbation in patients with tuberculosis
Scientific Reports (2020)