Key Points
-
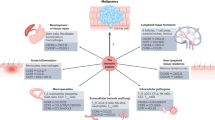
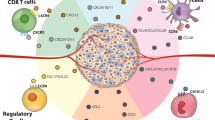
Chemokines are small, cytokine-like, secreted proteins (8–11 kDa) that regulate leukocyte transport by mediating the adhesion of leukocytes to endothelial cells, the initiation of transendothelial migration and tissue invasion. To date, we know of 24 human CC chemokines (CCL1–CCL28), 15 human CXC chemokines (CXCL1–16) and one each of the CX3C and C chemokine subclasses, which are represented by fractalkine (CX3CL1) and lymphotactin (XCL1), respectively.
-
The role of chemokines in tumour biology is complex. Besides their action on haematopoietic cells, recent studies have shown that chemokines also induce distinct effects in non-haematopoietic cells such as stromal and solid tumour cells. Chemokines can act as autocrine or paracrine growth factors, induce angiogenesis or angiostasis, regulate metastasis and have a role in the host's immune response against tumour cells.
-
Findings in experimental tumour models have shown that the introduction of chemokines such as CCL1 (I-309), CCL2 (monocyte chemoattractant protein-1; MCP-1), CCL3 (macrophage inflammatory protein-1a; MIP-1a), CCL5 (regulated upon activation normal T cell expressed and secreted, RANTES), CCL16 (human β CC chemokine 4; HCC-4), CCL19 (MIP-3b), CCL20 (MIP-3a), CCL21 (6Ckine), CXCL10 (interferon-γ inducible protein-10, IP-10) and XCL1 (lymphotactin) alone can induce tumour regression and immunity to subsequent tumour challenge.
-
Chemokines alone seem to show limited antitumour efficacy. However, new approaches are being developed that combine a chemoattractant (e.g. CCL19, CCL21, CXCL9, CXCL10, CXCL12 and XCL1) together with cytokines (interleukin-2 (IL-2), IL-12, granulocyte–macrophage colony-stimulating factor (GM-CSF)), which are known for their stimulating properties on T cells, natural killer (NK) cells or tumour antigen-pulsed dendritic cells (DCs).
-
Chemokines might act as potent natural adjuvants for experimental antitumour immunotherapy. Their combination with tumour peptide-pulsed DCs and direct coupling to tumour antigen or immunostimulatory cytokines results in synergistic antitumour activity. This is a way of reducing toxic side effects.
-
The combination of tumour antigen-releasing therapies (chemotherapy and radiation therapy) with chemokine delivery to sites of tumour antigen exposure, and the in vivo administration of 'DC-poietins' such as FTL3 ligand or GM-CSF and other activation molecules (IL-2, IL-12, CD40L and CpG), offers promising strategies to induce strong and long-lived antitumour immunity. However, side effects such as the induction of autoimmunity and the concept of leukocyte-mediated tumour-cell proliferation/survival and invasion should not be overlooked.
Abstract
Chemokines, a superfamilly of small cytokine-like molecules, regulate leukocyte transport in the body. In recent years, we have witnessed the transition of immunotherapeutic strategies from the laboratory to the bedside. Here, we review the role of chemokines in tumour biology and the development of the host's anti-tumour defence. We summarize the current knowledge of chemokine-receptor expression by relevant cellular components of the immune system and the role of their ligands in the organization of the antitumour immune response. Finally, we discuss recent findings which indicate that chemokines have therapeutic potential as adjuvants or treatments in antitumour immunotherapy, as well as remaining questions and perspectives for translating experimental evidence into clinical practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Homey, B. & Zlotnik, A. Chemokines in allergy. Curr. Opin. Immunol. 11, 626–634 (1999).
Zlotnik, A. & Yoshie, O. Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127 (2000).Introduces the new systematic classification for chemokines and describes their role in immunity.
Caux, C. et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin. Immunopathol. 22, 345–369 (2000).Describes chemokine receptor expression of distinct dendritic cell subsets, and their responsiveness and role in dendritic cell transport.
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Homey, B. et al. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J. Immunol. 164, 3465–3470 (2000).
Morales, J. et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc. Natl Acad. Sci. USA 96, 14470–14475 (1999).
Campbell, J. J. et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400, 776–780 (1999).
Campbell, D. J. & Butcher, E. C. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195, 135–141 (2002).
Nanki, T. et al. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J. Immunol. 165, 6590–6598 (2000).
Taub, D. D., Sayers, T. J., Carter, C. R. & Ortaldo, J. R. α and β chemokines induce NK cell migration and enhance NK-mediated cytolysis. J. Immunol. 155, 3877–3888 (1995).
Suzuki, Y., Rahman, M. & Mitsuya, H. Diverse transcriptional response of CD4+ T cells to stromal cell-derived factor (SDF)-1: cell survival promotion and priming effects of SDF-1 on CD4+ T cells. J. Immunol. 167, 3064–3073 (2001).
Bernardini, G. et al. I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood 96, 4039–4045 (2000).
Gupta, S. K., Lysko, P. G., Pillarisetti, K., Ohlstein, E. & Stadel, J. M. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J. Biol. Chem. 273, 4282–4287 (1998).
Salcedo, R. et al. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal-derived factor-1α. Am. J. Pathol. 154, 1125–1135 (1999).
Salcedo, R. et al. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J. Immunol. 166, 7571–7578 (2001).
Kleeff, J. et al. Detection and localization of Mip-3alpha/ LARC/Exodus, a macrophage proinflammatory chemokine, and its CCR6 receptor in human pancreatic cancer. Int. J. Cancer 81, 650–657 (1999).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Scotton, C. J., Wilson, J. L., Milliken, D., Stamp, G. & Balkwill, F. R. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 61, 4961–4965 (2001).References 18 and 19 show that tumour cells express functionally active chemokine receptors on their cell surface and provide evidence for the role for chemokine receptors in tumour cell migration and metastasis.
Schadendorf, D. et al. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J. Immunol. 151, 2667–2675 (1993).
Miyamoto, M. et al. Effect of interleukin-8 on production of tumor-associated substances and autocrine growth of human liver and pancreatic cancer cells. Cancer Immunol. Immunother. 47, 47–57 (1998).
Brew, R. et al. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine 12, 78–85 (2000).
Venkatakrishnan, G., Salgia, R. & Groopman, J. E. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J. Biol. Chem. 275, 6868–6875 (2000).
Balentien, E., Mufson, B. E., Shattuck, R. L., Derynck, R. & Richmond, A. Effects of MGSA/GROα on melanocyte transformation. Oncogene 6, 1115–1124 (1991).
Owen, J. D. et al. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine β and γ proteins. Int. J. Cancer 73, 94–103 (1997).
Arenberg, D. A. et al. Epithelial–neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J. Clin. Invest. 102, 465–472 (1998).
Ahuja, S. K. & Murphy, P. M. The CXC chemokines growth-regulated oncogene (GRO)α, GROβ, GROγ, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 271, 20545–20550 (1996).
Belperio, J. A. et al. CXC chemokines in angiogenesis. J. Leukoc. Biol. 68, 1–8 (2000).
Strieter, R. M. et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 270, 27348–27357 (1995).
Inoue, K. et al. Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin. Cancer Res. 6, 4874–4884 (2000).
Singh, R. K., Gutman, M., Radinsky, R., Bucana, C. D. & Fidler, I. J. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 54, 3242–3247 (1994).
Singh, R. K., Varney, M. L., Bucana, C. D. & Johansson, S. L. Expression of interleukin-8 in primary and metastatic malignant melanoma of the skin. Melanoma Res. 9, 383–387 (1999).
Liotta, L. A. & Kohn, E. C. The microenvironment of the tumour–host interface. Nature 411, 375–379 (2001).
Zeelenberg, I. S., Ruuls-Van Stalle, L. & Roos, E. Retention of CXCR4 in the endoplasmic reticulum blocks dissemination of a T cell hybridoma. J. Clin. Invest. 108, 269–277 (2001).
Murphy, P. M. Chemokines and the molecular basis of cancer metastasis. N. Engl. J. Med. 345, 833–835 (2001).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte–macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Asher, A. L. et al. Murine tumor cells transduced with the gene for tumor necrosis factor-α. Evidence for paracrine immune effects of tumor necrosis factor against tumors. J. Immunol. 146, 3227–3234 (1991).
Tahara, H. et al. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 54, 182–189 (1994).
Wang, J. M., Shen, W., Chertov, O., van Damme, J. & Oppenheimer, J. J. in Chemokines and Cancer (ed. Rollins, B. J.) 129–141 (Humana, Totowa, New Jersey, 1999).
Laning, J., Kawasaki, H., Tanaka, E., Luo, Y. & Dorf, M. E. Inhibition of in vivo tumor growth by the β chemokine, TCA3. J. Immunol. 153, 4625–4635 (1994).
Hirose, K. et al. Chemokine gene transfection into tumour cells reduced tumorigenicity in nude mice in association with neutrophilic infiltration. Br. J. Cancer 72, 708–714 (1995).
Nakashima, E. et al. A candidate for cancer gene therapy: MIP-1α gene transfer to an adenocarcinoma cell line reduced tumorigenicity and induced protective immunity in immunocompetent mice. Pharm. Res. 13, 1896–1901 (1996).
Mule, J. J. et al. RANTES secretion by gene-modified tumor cells results in loss of tumorigenicity in vivo: role of immune cell subpopulations. Hum. Gene Ther. 7, 1545–1553 (1996).
Giovarelli, M. et al. Tumor rejection and immune memory elicited by locally released LEC chemokine are associated with an impressive recruitment of APCs, lymphocytes, and granulocytes. J. Immunol. 164, 3200–3206 (2000).
Braun, S. E. et al. The CC chemokine CK β-11/MIP-3 β/ELC/ Exodus 3 mediates tumor rejection of murine breast cancer cells through NK cells. J. Immunol. 164, 4025–4031 (2000).
Fushimi, T., Kojima, A., Moore, M. A. & Crystal, R. G. Macrophage inflammatory protein 3α transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. J. Clin. Invest. 105, 1383–1393 (2000).
Kirk, C. J. et al. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 61, 2062–2070 (2001).Shows the efficacy of chemokine (CCL21)-transfected dendritic cells to induce the recruitment of leukocytes in vivo and to mediate the induction of antitumour immunity.
Sharma, S. et al. Secondary lymphoid tissue chemokine mediates T-cell-dependent antitumor responses in vivo. J. Immunol. 164, 4558–4563 (2000).
Vicari, A. P. et al. Antitumor effects of the mouse chemokine 6Ckine/SLC through angiostatic and immunological mechanisms. J. Immunol. 165, 1992–2000 (2000).
Luster, A. D. & Leder, P. IP-10, a-C–X–C-chemokine, elicits a potent thymus-dependent antitumor response in vivo. J. Exp. Med. 178, 1057–1065 (1993).
Cairns, C. M. et al. Lymphotactin expression by engineered myeloma cells drives tumor regression: mediation by CD4+ and CD8+ T cells and neutrophils expressing XCR1 receptor. J. Immunol. 167, 57–65 (2001).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).Reviews recent findings using dendritic-cell-based immunotherapy and gives an outlook for future directions.
Greten, T. F. & Jaffee, E. M. Cancer vaccines. J. Clin. Oncol. 17, 1047–1060 (1999).
Banchereau, J., Schuler-Thurner, B., Palucka, A. K. & Schuler, G. Dendritic cells as vectors for therapy. Cell 106, 271–274 (2001).
Nagira, M. et al. A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur. J. Immunol. 28, 1516–1523 (1998).
Kim, C. H. et al. CCR7 ligands, SLC/6Ckine/Exodus2/TCA4 and CKβ-11/MIP-3β/ELC, are chemoattractants for CD56+CD16− NK cells and late stage lymphoid progenitors. Cell. Immunol. 193, 226–235 (1999).
Hedrick, J. A. & Zlotnik, A. Identification and characterization of a novel βchemokine containing six conserved cysteines. J. Immunol. 159, 1589–1593 (1997).
Kelner, G. S. et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science 266, 1395–1399 (1994).
Kennedy, J. et al. Molecular cloning and functional characterization of human lymphotactin. J. Immunol. 155, 203–209 (1995).
Cao, X. et al. Lymphotactin gene-modified bone marrow dendritic cells act as more potent adjuvants for peptide delivery to induce specific antitumor immunity. J. Immunol. 161, 6238–6244 (1998).
Dilloo, D. et al. Combined chemokine and cytokine gene transfer enhances antitumor immunity. Nature Med. 2, 1090–1095 (1996).
Emtage, P. C. et al. Adenoviral vectors expressing lymphotactin and interleukin 2 or lymphotactin and interleukin 12 synergize to facilitate tumor regression in murine breast cancer models. Hum. Gene Ther. 10, 697–709 (1999).
Ma, X., Aste-Amezaga, M., Gri, G., Gerosa, F. & Trinchieri, G. Immunomodulatory functions and molecular regulation of IL-12. Chem. Immunol. 68, 1–22 (1997).
Trinchieri, G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 70, 83–243 (1998).
Voest, E. E. et al. Inhibition of angiogenesis in vivo by interleukin 12. J. Natl. Cancer Inst. 87, 581–586 (1995).
Narvaiza, I. et al. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-γ-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J. Immunol. 164, 3112–3122 (2000).
Palmer, K., Hitt, M., Emtage, P. C., Gyorffy, S. & Gauldie, J. Combined CXC chemokine and interleukin-12 gene transfer enhances antitumor immunity. Gene Ther. 8, 282–290 (2001).
Ruehlmann, J. M. et al. MIG (CXCL9) chemokine gene therapy combines with antibody–cytokine fusion protein to suppress growth and dissemination of murine colon carcinoma. Cancer Res. 61, 8498–8503 (2001).
Biragyn, A., Tani, K., Grimm, M. C., Weeks, S. & Kwak, L. W. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat. Biotechnol. 17, 253–258 (1999).Shows the efficacy of fusion proteins that combines a chemokine with tumour antigen to attract leukocytes in vivo and to induce protective antitumour immunity.
Tazi, A. et al. Evidence that granulocyte–macrophage-colony-stimulating factor regulates the distribution and differentiated state of dendritic cells/Langerhans cells in human lung and lung cancers. J. Clin. Invest. 91, 566–576 (1993).
Maas, R. A., Dullens, H. F. & Den Otter, W. Interleukin-2 in cancer treatment: disappointing or (still) promising? A review. Cancer Immunol. Immunother. 36, 141–148 (1993).
Nomura, T., Hasegawa, H., Kohno, M., Sasaki, M. & Fujita, S. Enhancement of anti-tumor immunity by tumor cells transfected with the secondary lymphoid tissue chemokine EBI-1-ligand chemokine and stromal cell-derived factor-1α chemokine genes. Int. J. Cancer 91, 597–606 (2001).Describes the enhanced efficacy of combining chemokines together with both T-cell (IL-2)- and dendritic-cell (GM-CSF)-activating cytokines for the immunotherapy of experimental tumours.
Morse, M. A., Clay, T. M., Hobeika, A. C., Mosca, P. J. & Lyerly, H. K. Surrogate markers of response to cancer immunotherapy. Expert Opin. Biol. Ther. 1, 153–158 (2001).
Glennie, M. J. & Johnson, P. W. Clinical trials of antibody therapy. Immunol. Today 21, 403–410 (2000).
Harjunpaa, A., Junnikkala, S. & Meri, S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand. J. Immunol. 51, 634–641 (2000).
Agha-Mohammadi, S. & Lotze, M. T. RegulaTable systems: applications in gene therapy and replicating viruses. J. Clin. Invest. 105, 1177–1183 (2000).
Kirkwood, J. M. et al. High- and low-dose interferon α-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J. Clin. Oncol. 18, 2444–2458 (2000).
Jager, D., Jager, E. & Knuth, A. Immune responses to tumour antigens: implications for antigen specific immunotherapy of cancer. J. Clin. Pathol. 54, 669–674 (2001).
Ockert, D., Schmitz, M., Hampl, M. & Rieber, E. P. Advances in cancer immunotherapy. Immunol. Today 20, 63–65 (1999).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).The authors review the links between cancer and inflammation. They propose that the inflammatory cells and cytokines that are found in tumours contribute to tumour growth, progression and immunosuppression, rather than to mount an effective host antitumour response.
Zou, W. et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nature Med. 7, 1339–1346 (2001).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Ansel, K. M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000).
Bottazzi, B., Walter, S., Govoni, D., Colotta, F. & Mantovani, A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J. Immunol. 148, 1280–1285 (1992).
Huang, S. et al. Expression of the JE/MCP-1 gene suppresses metastatic potential in murine colon carcinoma cells. Cancer Immunol. Immunother. 39, 231–238 (1994).
Nakashima, E. et al. Human MCAF gene transfer enhances the metastatic capacity of a mouse cachectic adenocarcinoma cell line in vivo. Pharm. Res. 12, 1598–1604 (1995).
Acknowledgements
We thank E. Bünemann for assistance, and S. Wagner and M. C. Dieu-Nosjean for discussions. We apologize to those colleagues whose work was not discussed due to space restrictions.
Author information
Authors and Affiliations
Corresponding author
Glossary
- ANGIOSTATIC
-
Angiostatic factors impair the formation of new capillary blood vessels, a process termed angiogenesis. Angiogenesis involves the proliferation, migration and differentiation of endothelial cells.
- MATRIX METALLOPROTEINASES (MMPs).
-
The catalytic activity of these enzymes is important in normal biology because of their role in morphogenesis, homeostasis and repair, and they are also implicated in inflammation and disease. Beyond their role in the turnover and degradation of extracellular-matrix proteins, MMPs also process, activate and deactivate various soluble factors.
- ACTIN POLYMERIZATION
-
Part of the intracellular cytoskeletal rearrangement that is the prerequisite for cell motility and migration.
- TNM CLASSIFICATION
-
A staging system for cancer patients. T1–4 indicates primary tumour size. N0 indicates no evidence of lymph node metastasis, N+ indicates regional lymph node metastasis, M0 indicates no distant metastasis and M1 indicates distant metastasis.
- TYPE 1 MEMORY T CELLS
-
(TH1 cells). These are CD4+ T-helper cells, which predominantly produce interferon-γ.
- DC-POIETIN
-
A factor/cytokine that is able to expand the number of circulating dendritic cells.
- MICROARRAY ANALYSES
-
A powerful tool for identifying genes that are associated with complex biological phenomena. Microarrays contain human cDNAs of known and unknown sequences, which are printed on glass slides using high-speed robotics. These DNA 'chips' are used to quantitatively monitor differential expression of the human genes using a highly sensitive two-colour hybridization assay.
- PROTEOMICS
-
A promising approach for the identification of proteins and biochemical pathways that are involved in tumorigenesis. In an effort to discover such tumour-associated proteins and pathways, tumour protein lysates are subjected to two-dimensional electrophoresis and mass spectrometry and are analysed using large protein databases.
- FTL3 LIGAND
-
A cytokine that binds to the FLT3/FLK2 tyrosine kinase receptor, stimulates the proliferation of defined subpopulations of bone-marrow cells and increases the number of circulating dendritic cells.
- CD40 LIGAND
-
(CD40L, CD154). A member of the tumour-necrosis factor family of cell-surface molecules and mediates contact-dependent signals that are delivered by CD4+ T-helper cells to CD40+ target cells.
- PREDC2
-
(pDC2/IPC (type 1 interferon-producing cell)). Represents a subset of immature CD4+CD3−CD11c− plasmacytoid dendritic cells, which are also known as natural IPCs.
- CPG MOTIFS
-
Immunostimulatory sequences of DNA that are potent mediators of TH1 and cytotoxic T-lymphocyte responses to antigens.
Rights and permissions
About this article
Cite this article
Homey, B., Müller, A. & Zlotnik, A. Chemokines: agents for the immunotherapy of cancer?. Nat Rev Immunol 2, 175–184 (2002). https://doi.org/10.1038/nri748
Issue Date:
DOI: https://doi.org/10.1038/nri748
This article is cited by
-
Targeted inhibition of CX3CL1 limits podocytes ferroptosis to ameliorate cisplatin-induced acute kidney injury
Molecular Medicine (2023)
-
Mechanism and therapeutic implications of pomalidomide-induced immune surface marker upregulation in EBV-positive lymphomas
Scientific Reports (2023)
-
Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases
Nature Biomedical Engineering (2020)
-
Phase I study of an active immunotherapy for asymptomatic phase Lymphoplasmacytic lymphoma with DNA vaccines encoding antigen-chemokine fusion: study protocol
BMC Cancer (2018)
-
Human MSCs promotes colorectal cancer epithelial–mesenchymal transition and progression via CCL5/β-catenin/Slug pathway
Cell Death & Disease (2017)