Key Points
-
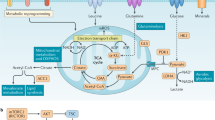
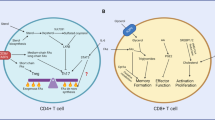
Naive, effector and memory T cells have distinct metabolic profiles that are crucial for their maintenance and function.
-
A complex network of immune cell-specific transcription factors, cytokines and canonical regulators of cellular metabolism regulate the diversity of effector and memory T cells.
-
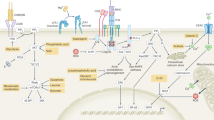
The T cell receptor-dependent transcription factors interferon-regulatory factor 4 (IRF4) and MYC coordinately induce gene networks that are required for metabolic reprogramming and for sustaining aerobic glycolysis during periods of rapid clonal expansion.
-
Clonal competition and the selection of high-affinity T cell clones during a primary immune response are driven by metabolic fitness.
-
Common cytokine receptor γ-chain cytokines modulate metabolic profiles during effector and memory T cell differentiation and maintenance.
-
Immune cell-specific transcription factors sense and integrate metabolic signals with immunological cues to appropriately regulate adaptive immune responses.
Abstract
During an immune response, cytokines and transcription factors regulate the differentiation and function of effector and memory T cells. At the same time, T cell metabolism undergoes dynamic and differentiation-stage-specific changes that are required for initial T cell activation, rapid proliferation and the acquisition of effector functions. Similarly, during the resolution of an immune response, metabolic regulation is crucial for restraining inflammatory responses and promoting peripheral tolerance, and it is required for the long-term maintenance of memory T cells. T cell receptor (TCR)-induced transcription factors, in particular MYC and interferon-regulatory factor 4 (IRF4), cooperate with canonical nutrient-sensing pathways to integrate antigen-specific and metabolic signals to appropriately modulate adaptive immune responses. In this Review, we focus on the emerging evidence that T cell differentiation and metabolism are closely linked and synchronized by immune cell-specific cytokines and transcription factors that are induced by TCR signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
Pollizzi, K. N. & Powell, J. D. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat. Rev. Immunol. 14, 435–446 (2014).
MacIver, N. J., Michalek, R. D. & Rathmell, J. C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283 (2013).
Wang, R. & Green, D. R. Metabolic checkpoints in activated T cells. Nat. Immunol. 13, 907–915 (2012).
Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 12, 325–338 (2012).
Finlay, D. & Cantrell, D. A. Metabolism, migration and memory in cytotoxic T cells. Nat. Rev. Immunol. 11, 109–117 (2011).
O'Shea, J. J. & Paul, W. E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327, 1098–1102 (2010).
Yamane, H. & Paul, W. E. Cytokines of the γC family control CD4+ T cell differentiation and function. Nat. Immunol. 13, 1037–1044 (2012).
Carbone, F. R., Mackay, L. K., Heath, W. R. & Gebhardt, T. Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Curr. Opin. Immunol. 25, 329–333 (2013).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Jiang, X. et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231 (2012).
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Chang, J. T., Wherry, E. J. & Goldrath, A. W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 15, 1104–1115 (2014).
Brustle, A. et al. The development of inflammatory TH17 cells requires interferon-regulatory factor 4. Nat. Immunol. 8, 958–966 (2007).
Staudt, V. et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33, 192–202 (2010).
Kwon, H. et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity 31, 941–952 (2009).
Cretney, E. et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–311 (2011).
Man, K. et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 14, 1155–1165 (2013). This study demonstrates that IRF4 regulates clonal expansion and effector function of CD8+ T cells. In particular, IRF4 was shown to be required for maintaining the expression of genes required of the glycolytic pathway and modulating the expression of key regulators of cellular metabolism, including FOXO1, HIF1α and PPARγ.
Yao, S. et al. Interferon regulatory factor 4 sustains CD8+ T cell expansion and effector differentiation. Immunity 39, 833–845 (2013).
Grusdat, M. et al. IRF4 and BATF are critical for CD8+ T-cell function following infection with LCMV. Cell Death Differ. 21, 1050–1060 (2014).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Carr, E. L. et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 185, 1037–1044 (2010).
Sinclair, L. V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508 (2013).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013). This study shows that aerobic glycolysis is directly required for efficient cytokine production by effector T cells by blocking glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-mediated inhibition of Ifng and Il2 mRNA translation.
Blagih, J. et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42, 41–54 (2015).
Rolf, J. et al. AMPKα1: a glucose sensor that controls CD8 T-cell memory. Eur. J. Immunol. 43, 889–896 (2013).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Lochner, M., Berod, L. & Sparwasser, T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 36, 81–91 (2015).
Sukumar, M. et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 123, 4479–4488 (2013). This study demonstrates that restricting glycolysis or the duration of glycolysis in effector T cells enhances memory T cell development and increases the expression of memory cell-specific transcription factors such as TCF7, LEF1 and BCL-6.
Efeyan, A. et al. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493, 679–683 (2013).
Dowling, R. J. et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328, 1172–1176 (2010).
Sancak, Y. et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 (2008).
Sancak, Y. et al. Ragulator–Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 (2010).
Bar-Peled, L., Schweitzer, L. D., Zoncu, R. & Sabatini, D. M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196–1208 (2012).
Gwinn, D. M. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008).
Inoki, K., Zhu, T. & Guan, K. L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003).
Inoki, K. et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 (2006).
Brugarolas, J. et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904 (2004).
Bernardi, R. et al. PML inhibits HIF-1α translation and neoangiogenesis through repression of mTOR. Nature 442, 779–785 (2006).
Li, Y. et al. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J. Biol. Chem. 282, 35803–35813 (2007).
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and TReg cells. J. Exp. Med. 208, 1367–1376 (2011).
Dang, E. V. et al. Control of TH17/TReg balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011). References 41 and 42 demonstrate that signalling via mTORC1 and expression of HIF1α are required for T H 17 cell differentiation by promoting glycolytic activity. They further show that HIF1α directly binds to the Rorc locus (which encodes RORγt) to activate its transcription, while mediating FOXP3 protein degradation, thus stabilizing T H 17 cell differentiation.
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Klotz, L. et al. The nuclear receptor PPAR γ selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 206, 2079–2089 (2009).
Gerriets, V. A. et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125, 194–207 (2015).
Harris, T. J. et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 179, 4313–4317 (2007).
Berod, L. et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20, 1327–1333 (2014).
Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 (2010).
Semenza, G. L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Lee, W. H. & Kim, S. G. AMPK-dependent metabolic regulation by PPAR agonists. PPAR Res. 2010, 549101 (2010).
Bensinger, S. J. & Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454, 470–477 (2008).
Tontonoz, P., Nagy, L., Alvarez, J. G., Thomazy, V. A. & Evans, R. M. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93, 241–252 (1998).
Tontonoz, P., Hu, E. & Spiegelman, B. M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79, 1147–1156 (1994).
Kliewer, S. A. et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl Acad. Sci. USA 94, 4318–4323 (1997).
Pascual, G. et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature 437, 759–763 (2005).
Jiang, C., Ting, A. T. & Seed, B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391, 82–86 (1998).
Yang, X. Y. et al. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor γ (PPARγ) agonists. PPARγ co-association with transcription factor NFAT. J. Biol. Chem. 275, 4541–4544 (2000).
Wang, L. H. et al. Transcriptional inactivation of STAT3 by PPARγ suppresses IL-6-responsive multiple myeloma cells. Immunity 20, 205–218 (2004).
Soroosh, P. et al. Oxysterols are agonist ligands of RORγt and drive TH17 cell differentiation. Proc. Natl Acad. Sci. USA 111, 12163–12168 (2014).
Xiao, S. et al. Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 40, 477–489 (2014).
Sauer, S. et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl Acad. Sci. USA 105, 7797–7802 (2008).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).
Liu, G., Yang, K., Burns, S., Shrestha, S. & Chi, H. The S1P1-mTOR axis directs the reciprocal differentiation of TH1 and TReg cells. Nat. Immunol. 11, 1047–1056 (2010).
Procaccini, C. et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity 33, 929–941 (2010).
Zeng, H. et al. mTORC1 couples immune signals and metabolic programming to establish TReg-cell function. Nature 499, 485–490 (2013).
Vahl, J. C. et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity 41, 722–736 (2014).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Kidani, Y. et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 14, 489–499 (2013).
Rao, R. R., Li, Q., Gubbels Bupp, M. R. & Shrikant, P. A. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8+ T cell differentiation. Immunity 36, 374–387 (2012).
Chen, C. C. et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev. Cell 18, 592–604 (2010).
Kim, M. V., Ouyang, W., Liao, W., Zhang, M. Q. & Li, M. O. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity 39, 286–297 (2013).
Rao, R. R., Li, Q., Odunsi, K. & Shrikant, P. A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 32, 67–78 (2010).
Hess Michelini, R., Doedens, A. L., Goldrath, A. W. & Hedrick, S. M. Differentiation of CD8 memory T cells depends on Foxo1. J. Exp. Med. 210, 1189–1200 (2013). References 74 and 75 link the mTOR target and metabolic regulator FOXO1 with T-bet and EOMES in a pathway that influences the balance between effector and memory T cell differentiation.
Lucas, C. L. et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 15, 88–97 (2014).
Shrestha, S. et al. Tsc1 promotes the differentiation of memory CD8+ T cells via orchestrating the transcriptional and metabolic programs. Proc. Natl Acad. Sci. USA 111, 14858–14863 (2014).
Pollizzi, K. N. et al. mTORC1 and mTORC2 selectively regulate CD8+ T cell differentiation. J. Clin. Invest. 125, 2090–2108 (2015).
Yang, K. et al. T cell exit from quiescence and differentiation into TH2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity 39, 1043–1056 (2013).
Busch, D. H. & Pamer, E. G. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189, 701–710 (1999).
Malherbe, L., Hausl, C., Teyton, L. & McHeyzer-Williams, M. G. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity 21, 669–679 (2004).
Price, D. A. et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 202, 1349–1361 (2005).
Savage, P. A., Boniface, J. J. & Davis, M. M. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity 10, 485–492 (1999).
Wensveen, F. M. et al. Apoptosis threshold set by Noxa and Mcl-1 after T cell activation regulates competitive selection of high-affinity clones. Immunity 32, 754–765 (2010).
Zehn, D., Lee, S. Y. & Bevan, M. J. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214 (2009).
Teixeiro, E. et al. Different T cell receptor signals determine CD8+ memory versus effector development. Science 323, 502–505 (2009).
Smith-Garvin, J. E. et al. T-cell receptor signals direct the composition and function of the memory CD8+ T-cell pool. Blood 116, 5548–5559 (2010).
Marchingo, J. M. et al. T cell signaling. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science 346, 1123–1127 (2014).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011). This paper demonstrates that the transcription factor MYC directly regulates T cell proliferation by coordinating aspects of activation-induced metabolic reprogramming, including the coupling of glucose and glutamine metabolism with various biosynthetic pathways.
Duvel, K. et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 (2010).
Finlay, D. K. et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453 (2012). This paper shows that IL-2, PDK1–AKT1–mTORC1 signalling and HIF1α are all required for inducing aerobic glycolysis following antigen recognition and that they promote the effector differentiation of CD8+ T cells.
Doedens, A. L. et al. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 14, 1173–1182 (2013).
Nayar, R. et al. Graded levels of IRF4 regulate CD8+ T cell differentiation and expansion, but not attrition, in response to acute virus infection. J. Immunol. 192, 5881–5893 (2014).
Kurachi, M. et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat. Immunol. 15, 373–383 (2014).
Glasmacher, E. et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1–IRF complexes. Science 338, 975–980 (2012).
Ciofani, M. et al. A validated regulatory network for TH17 cell specification. Cell 151, 289–303 (2012).
Li, P. et al. BATF–JUN is critical for IRF4-mediated transcription in T cells. Nature 490, 543–546 (2012).
Vasanthakumar, A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16, 276–285 (2015).
Chou, C. et al. c-Myc-induced transcription factor AP4 is required for host protection mediated by CD8+ T cells. Nat. Immunol. 15, 884–893 (2014).
Pastorino, J. G., Shulga, N. & Hoek, J. B. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 277, 7610–7618 (2002).
Majewski, N., Nogueira, V., Robey, R. B. & Hay, N. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol. Cell. Biol. 24, 730–740 (2004).
Mihara, M. et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577–590 (2003).
Zhao, Y. et al. Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J. Biol. Chem. 283, 36344–36353 (2008).
Bensaad, K. et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126, 107–120 (2006).
Rathmell, J. C. et al. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell. Biol. 23, 7315–7328 (2003).
Yamaguchi, H. & Wang, H. G. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 20, 7779–7786 (2001).
Alves, N. L. et al. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity 24, 703–716 (2006). This study indicates that under conditions of glucose limitation, levels of the pro-survival molecule MCL1 decline and the activity of interacting BH3-only protein NOXA confers susceptibility to apoptosis in activated T cells. MCL1 levels could be restored by the addition of glucose, demonstrating that proteins involved in regulating cell death are sensitive to metabolic signals.
Pierson, W. et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat. Immunol. 14, 959–965 (2013).
Rochman, Y., Spolski, R. & Leonard, W. J. New insights into the regulation of T cells by γC family cytokines. Nat. Rev. Immunol. 9, 480–490 (2009).
Kim, M. T. & Harty, J. T. Impact of inflammatory cytokines on effector and memory CD8+ T cells. Frontiers Immunol. 5, 295 (2014).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Kalia, V. et al. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103 (2010).
Pipkin, M. E. et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32, 79–90 (2010).
Williams, M. A., Tyznik, A. J. & Bevan, M. J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441, 890–893 (2006).
Takemoto, N., Intlekofer, A. M., Northrup, J. T., Wherry, E. J. & Reiner, S. L. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 177, 7515–7519 (2006).
Joshi, N. S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007).
Macintyre, A. N. et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity 34, 224–236 (2011).
Schluns, K. S. & Lefrancois, L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3, 269–279 (2003).
Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678 (2000).
Surh, C. D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29, 848–862 (2008).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
O'Sullivan, D. et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014). References 121 and 122 demonstrate that the memory-promoting cytokine IL-15 is involved in inducing the expression of genes that are required for promoting mitochondrial biogenesis, fatty acid transport, de novo FAS and FAO that promote the maintenance and longevity of memory T cells.
Wofford, J. A., Wieman, H. L., Jacobs, S. R., Zhao, Y. & Rathmell, J. C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111, 2101–2111 (2008).
Jacobs, S. R. et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 180, 4476–4486 (2008).
Cui, G. et al. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell 161, 750–761 (2015). This recent study shows that IL-7 is required for inducing the expression of the glycerol transporter aquaporin 9 and triacylglycerol synthases. It further demonstrates that the synthesis of triacylglycerols, glycerol and free fatty acids is an important source of neutral lipids and is required for the survival of CD8+ memory T cells.
Cui, W., Liu, Y., Weinstein, J. S., Craft, J. & Kaech, S. M. An interleukin-21–interleukin-10–STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Siegel, A. M. et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity 35, 806–818 (2011).
Gough, D. J. et al. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 324, 1713–1716 (2009).
Wegrzyn, J. et al. Function of mitochondrial Stat3 in cellular respiration. Science 323, 793–797 (2009). References 128 and 129 demonstrate that the transcription factor STAT3, which is important for memory T cell differentiation, has functions outside of classical transcriptional regulation and can be found in the mitochondria, where it regulates the function of complexes I and II of the electron transport chain and OXPHOS.
Laplante, M. & Sabatini, D. M. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 126, 1713–1719 (2013).
Powell, J. D. & Delgoffe, G. M. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 33, 301–311 (2010).
Cipolletta, D. et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue TReg cells. Nature 486, 549–553 (2012).
Cunard, R. et al. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J. Immunol. 168, 2795–2802 (2002).
Reilly, C. M. et al. Inhibition of mesangial cell nitric oxide in MRL/lpr mice by prostaglandin J2 and proliferator activation receptor-γ agonists. J. Immunol. 164, 1498–1504 (2000).
Chung, S. W., Kang, B. Y. & Kim, T. S. Inhibition of interleukin-4 production in CD4+ T cells by peroxisome proliferator-activated receptor-γ (PPAR-γ) ligands: involvement of physical association between PPAR-γ and the nuclear factor of activated T cells transcription factor. Mol. Pharmacol. 64, 1169–1179 (2003). References 133–135 suggest an important link between PPARγ-mediated regulation of lipid metabolism and the inhibition of inflammatory gene expression and cytokine production by CD4+ T cells.
Ichii, H. et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat. Immunol. 3, 558–563 (2002).
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Oestreich, K. J. et al. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat. Immunol. 15, 957–964 (2014).
Intlekofer, A. M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
van Grevenynghe, J. et al. Transcription factor FOXO3a controls the persistence of memory CD4+ T cells during HIV infection. Nature Med. 14, 266–274 (2008).
Staron, M. M. et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity 41, 802–814 (2014).
Eguchi, J. et al. Transcriptional control of adipose lipid handling by IRF4. Cell. Metabolism 13, 249–259 (2011).
Kong, X. et al. IRF4 is a key thermogenic transcriptional partner of PGC-1α. Cell 158, 69–83 (2014). References 142 and 143 show that IRF4 — which is typically considered to be a haematopoietic cell-specific transcriptional regulator — regulates lipid metabolism in adipocytes and is crucial for the thermogenic capacity of brown adipose tissue in response to environmental cold via cooperative transcriptional binding with PPARγ coactivator 1α (PGC1α).
Ortega-Molina, A. et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab. 15, 382–394 (2012).
Odegaard, J. I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
LaPensee, C. R., Lin, G., Dent, A. L. & Schwartz, J. Deficiency of the transcriptional repressor B cell lymphoma 6 (Bcl6) is accompanied by dysregulated lipid metabolism. PloS ONE 9, e97090 (2014).
Eijkelenboom, A. & Burgering, B. M. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 14, 83–97 (2013).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Acknowledgements
The authors thank the members of the Kallies laboratory for their various inputs. This work was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia (A.K.), by fellowships from the Sylvia and Charles Viertel Foundation (A.K.) and the Victoria Cancer Council (K.M.), and by the Victorian State Government Operational Infrastructure Support and Australian Government NHMRC Independent Research Institute Infrastructure Support scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Glossary
- Catabolic state
-
A state of metabolism characterized by the breakdown of complex substances into simpler ones, which is often accompanied by ATP production. Examples include the oxidation of fatty acids and amino acids.
- Anabolic state
-
A state of metabolism characterized by chemical reactions that build complex molecules from simpler units, consuming energy in the process.
- Oxidative phosphorylation
-
A two-step metabolic pathway that produces ATP from the oxidation of nutrients and the transfer of electrons. First, pyruvate and fatty acids are converted into acetyl-CoA, which enters the tricarboxylic acid cycle, yielding free electrons carried by NADH and FADH2. Second, the electrons enter the electron transport chain, resulting in the movement of protons out of the mitochondrial matrix and the synthesis of ATP.
- Fatty acid oxidation
-
(FAO). An important metabolic process used to derive energy through the mobilization and oxidation of fatty acids, mainly in the mitochondrial matrix. FAO is positively and negatively regulated by AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR), respectively.
- Glycolysis
-
Oxygen-independent metabolism of glucose and other sugars into pyruvate to produce energy in the form of ATP and intermediate substrates for other metabolic pathways. When combined with oxygen-dependent enzyme reactions, a more complete breakdown occurs, producing more energy.
- Glutaminolysis
-
A series of biochemical reactions in which the amino acid glutamine is degraded to glutamate and then to α-ketoglutarate for further metabolism in the tricarboxylic acid cycle.
- mTOR complex 1
-
(mTORC1). A complex consisting of: mammalian target of rapamycin (mTOR), which is a serine/threonine kinase; regulatory-associated protein of mTOR (RAPTOR); proline-rich AKT1 substrate of 40 kDa (PRAS40), which is an mTORC1 inhibitor; mLST8 (also known as GβL), which is of unknown function; and DEP domain-containing mTOR-interacting protein (DEPTOR), which is an mTOR inhibitor.
- Tricarboxylic acid cycle
-
Also known as Krebs cycle. Acetyl-CoA derived from glucose or fat breakdown is further metabolized to generate reduced energy equivalents that are used by mitochondrial oxidative phosphorylation to generate ATP.
- Oxysterols
-
Natural molecules derived from cholesterol oxidation.
- T cell exhaustion
-
A state in which effector T cells have an impaired ability to carry out their functions, such as cytotoxicity and cytokine secretion, owing to chronic stimulation by antigens. It is characterized by increased surface expression of inhibitory receptors such as programmed cell death protein 1.
Rights and permissions
About this article
Cite this article
Man, K., Kallies, A. Synchronizing transcriptional control of T cell metabolism and function. Nat Rev Immunol 15, 574–584 (2015). https://doi.org/10.1038/nri3874
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3874
This article is cited by
-
Insights from a 30-year journey: function, regulation and therapeutic modulation of PD1
Nature Reviews Immunology (2023)
-
Immune inhibitory receptor-mediated immune response, metabolic adaptation, and clinical characterization in patients with COVID-19
Scientific Reports (2023)
-
Zinc finger proteins: insights into the transcriptional and post transcriptional regulation of immune response
Molecular Biology Reports (2021)
-
Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH
Nature (2021)
-
Signaling networks in immunometabolism
Cell Research (2020)