Key Points
-
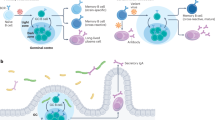
Germinal centre-independent memory B cells are generated from CD38+GL7+ activated B cells. These memory B cells may maintain broad reactivity to the activating pathogen.
-
B1a and B1b cells can generate T cell-independent memory B cells.
-
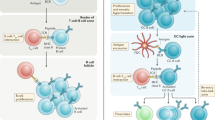
IgG+ and IgM+ memory B cells have a distinct function. IgG+ memory B cells preferentially differentiate into plasma cells, whereas IgM+ memory B cells predominantly enter the germinal centre reaction.
-
Bona fide IgE+ memory B cells are not present or, if they exist, they are present only as a small population.
-
Stimulation history, rather than the unique properties of the IgG cytoplasmic tail, is essential for exerting the rapid responses of IgG+ memory B cells.
-
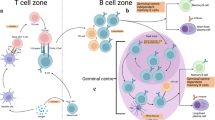
Memory T follicular helper cells support memory B cell responses. During this step, memory B cells are crucial antigen-presenting cells.
Abstract
The immune system can remember a previously experienced pathogen and can evoke an enhanced response to reinfection that depends on memory lymphocyte populations. Recent advances in tracking antigen-experienced memory B cells have revealed the existence of distinct classes of cells that have considerable functional differences. Some of these differences seem to be determined by the stimulation history during memory cell formation. To induce rapid recall antibody responses, the contributions of other types of cells, such as memory T follicular helper cells, have also now begun to be appreciated. In this Review, we discuss these and other recent advances in our understanding of memory B cells, focusing on the underlying mechanisms that are required for rapid and effective recall antibody responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Jacob, J., Kelsoe, G., Rajewsky, K. & Weiss, U. Intraclonal generation of antibody mutants in germinal centres. Nature 354, 389–392 (1991).
Berek, C., Berger, A. & Apel, M. Maturation of the immune response in germinal centers. Cell 67, 1121–1129 (1991).
Liu, Y. J. et al. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity 4, 241–250 (1996).
Anderson, S. M., Tomayko, M. M., Ahuja, A., Haberman, A. M. & Shlomchik, M. J. New markers for murine memory B cells that define mutated and unmutated subsets. J. Exp. Med. 204, 2103–2114 (2007).
Taylor, J. J., Pape, K. A. & Jenkins, M. K. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J. Exp. Med. 209, 597–606 (2012).
Kaji, T. et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J. Exp. Med. 209, 2079–2097 (2012). References 6 and 7 show that long-lived memory B cells can be generated independently of germinal centres.
Klein, U., Kuppers, R. & Rajewsky, K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood 89, 1288–1298 (1997).
Dogan, I. et al. Multiple layers of B cell memory with different effector functions. Nature Immunol. 10, 1292–1299 (2009).
Pape, K. A., Taylor, J. J., Maul, R. W., Gearhart, P. J. & Jenkins, M. K. Different B cell populations mediate early and late memory during an endogenous immune response. Science 331, 1203–1207 (2011). References 9 and 10 indicate that IgM+ and IgG+ memory B cells have different functions upon restimulation.
Takemori, T., Kaji, T., Takahashi, Y., Shimoda, M. & Rajewsky, K. Generation of memory B cells inside and outside germinal centers. Eur. J. Immunol. 44, 1258–1264 (2014).
Schwickert, T. A. et al. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J. Exp. Med. 208, 1243–1252 (2011).
Allen, C. D., Okada, T., Tang, H. L. & Cyster, J. G. Imaging of germinal center selection events during affinity maturation. Science 315, 528–531 (2007).
Qi, H., Cannons, J. L., Klauschen, F., Schwartzberg, P. L. & Germain, R. N. SAP-controlled T–B cell interactions underlie germinal centre formation. Nature 455, 764–769 (2008).
Linterman, M. A. et al. IL-21 acts directly on B cells to regulate BCL-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Dent, A. L. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276, 589–592 (1997).
Shulman, Z. et al. T follicular helper cell dynamics in germinal centers. Science 341, 673–677 (2013).
Shulman, Z. et al. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science 345, 1058–1062 (2014).
Gitlin, A. D., Shulman, Z. & Nussenzweig, M. C. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature 509, 637–640 (2014).
Liu, D. et al. T–B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature 517, 214–218 (2015).
Xu, H. et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 496, 523–527 (2013).
De Silva, N. S. & Klein, U. Dynamics of B cells in germinal centres. Nature Rev. Immunol. http://dx.doi.org/10.1038/nri3804 (2015).
Fischer, S. F. et al. Proapoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody-forming cells. Blood 110, 3978–3984 (2007).
Clybouw, C. et al. Regulation of memory B-cell survival by the BH3-only protein Puma. Blood 118, 4120–4128 (2011).
Takahashi, Y., Ohta, H. & Takemori, T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity 14, 181–192 (2001).
Alugupalli, K. R. et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21, 379–390 (2004).
Obukhanych, T. V. & Nussenzweig, M. C. T-independent type II immune responses generate memory B cells. J. Exp. Med. 203, 305–310 (2006).
Yang, Y. et al. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc. Natl Acad. Sci. USA 109, 5388–5393 (2012).
Montecino-Rodriguez, E. & Dorshkind, K. B-1 B cell development in the fetus and adult. Immunity 36, 13–21 (2012).
Haas, K. M., Poe, J. C., Steeber, D. A. & Tedder, T. F. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23, 7–18 (2005).
Tarlinton, D. & Good-Jacobson, K. Diversity among memory B cells: origin, consequences, and utility. Science 341, 1205–1211 (2013).
Aiba, Y. et al. Preferential localization of IgG memory B cells adjacent to contracted germinal centers. Proc. Natl Acad. Sci. USA 107, 12192–12197 (2010).
Berek, C. The development of B cells and the B-cell repertoire in the microenvironment of the germinal center. Immunol. Rev. 126, 5–19 (1992).
Zuccarino-Catania, G. V. et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nature Immunol. 15, 631–637 (2014).
Wang, N. S. et al. Divergent transcriptional programming of class-specific B cell memory by T-bet and RORα. Nature Immunol. 13, 604–611 (2012). This paper shows that T-bet or RORα are highly expressed in IgG2a+ or IgA+ memory B cells, respectively, and that these transcription factors are crucial for the survival of each memory B cell subset.
Gould, H. J. & Sutton, B. J. IgE in allergy and asthma today. Nature Rev. Immunol. 8, 205–217 (2008).
Xiong, H., Dolpady, J., Wabl, M., Curotto de Lafaille, M. A. & Lafaille, J. J. Sequential class switching is required for the generation of high affinity IgE antibodies. J. Exp. Med. 209, 353–364 (2012).
He, J.-S. et al. The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. J. Exp. Med. 210, 2755–2771 (2013).
Yang, Z., Sullivan, B. M. & Allen, C. D. Fluorescent in vivo detection reveals that IgE+ B cells are restrained by an intrinsic cell fate predisposition. Immunity 36, 857–872 (2012).
Talay, O. et al. IgE+ memory B cells and plasma cells generated through a germinal-center pathway. Nature Immunol. 13, 396–404 (2012). Using IgE reporter mice that were generated independently, references 38 and 39 show the lack of bona fide IgE+ memory B cells, whereas reference 40 identifies IgE+ memory B cells.
Brightbill, H. D. et al. Antibodies specific for a segment of human membrane IgE deplete IgE-producing B cells in humanized mice. J. Clin. Invest. 120, 2218–2229 (2010).
Luckey, C. J. et al. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc. Natl Acad. Sci. USA 103, 3304–3309 (2006).
Graef, P. et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8+ central memory T cells. Immunity 41, 116–126 (2014).
Barrington, R. A., Pozdnyakova, O., Zafari, M. R., Benjamin, C. D. & Carroll, M. C. B lymphocyte memory: role of stromal cell complement and FcγRIIB receptors. J. Exp. Med. 196, 1189–1199 (2002).
Hikida, M. et al. PLC-γ2 is essential for formation and maintenance of memory B cells. J. Exp. Med. 206, 681–689 (2009).
Maruyama, M., Lam, K. P. & Rajewsky, K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature 407, 636–642 (2000).
Carrington, E. M. et al. BH3 mimetics antagonizing restricted prosurvival BCL-2 proteins represent another class of selective immune modulatory drugs. Proc. Natl Acad. Sci. USA 107, 10967–10971 (2010).
Benson, M. J. et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 180, 3655–3659 (2008).
Cremasco, V. et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nature Immunol. 15, 973–981 (2014).
Yu, X. et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature 455, 532–536 (2008).
Engels, N. et al. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nature Immunol. 10, 1018–1025 (2009).
Liu, W. et al. The scaffolding protein synapse-associated protein 97 is required for enhanced signaling through isotype-switched IgG memory B cell receptors. Sci. Signal. 5, ra54 (2012).
Kometani, K. et al. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity 39, 136–147 (2013). This paper shows that antigen experience confers IgG1+ memory B cells with a predisposition to differentiate into plasma cells, which can be partly attributed to the repression of BACH2 expression in IgG1+ memory B cells.
Kallies, A. et al. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity 26, 555–566 (2007).
Martin, S. W. & Goodnow, C. C. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nature Immunol. 3, 182–188 (2002).
Chan, T. D. et al. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J. Immunol. 183, 3139–3149 (2009).
Hebeis, B. J. et al. Activation of virus-specific memory B cells in the absence of T cell help. J. Exp. Med. 199, 593–602 (2004).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000).
Kim, C. H. et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 193, 1373–1381 (2001).
MacLeod, M. K. et al. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol. 186, 2889–2896 (2011).
Weber, J. P., Fuhrmann, F. & Hutloff, A. T-follicular helper cells survive as long-term memory cells. Eur. J. Immunol. 42, 1981–1988 (2012).
Choi, Y. S. et al. BCL6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J. Immunol. 190, 4014–4026 (2013).
Hale, J. S. et al. Distinct memory CD4+ T cells with commitment to T follicular helper-and T helper 1-cell lineages are generated after acute viral infection. Immunity 38, 805–817 (2013).
Ise, W. et al. Memory B cells contribute to rapid BCL6 expression by memory follicular helper T cells. Proc. Natl Acad. Sci. USA 111, 11792–11797 (2014).
Shimoda, M., Li, T., Pihkala, J. P. & Koni, P. A. Role of MHC class II on memory B cells in post-germinal center B cell homeostasis and memory response. J. Immunol. 176, 2122–2133 (2006).
Chevalier, N. et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J. Immunol. 186, 5556–5568 (2011).
Heesters, B. A. et al. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity 38, 1164–1175 (2013).
Weill, J.-C., Le Gallou, S., Hao, Y. & Reynaud, C.-A. Multiple players in mouse B cell memory. Curr. Opin. Immunol. 25, 334–338 (2013).
Kasturi, S. P. et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011).
Schwickert, T. A., Alabyev, B., Manser, T. & Nussenzweig, M. C. Germinal center reutilization by newly activated B cells. J. Exp. Med. 206, 2907–2914 (2009).
Moir, S. et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205, 1797–1805 (2008).
Kardava, L. et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J. Clin. Invest. 121, 2614–2624 (2011).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Charles, E. D. et al. Clonal B cells in patients with hepatitis C virus-associated mixed cryoglobulinemia contain an expanded anergic CD21low B-cell subset. Blood 117, 5425–5437 (2011).
Weiss, G. E. et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J. Immunol. 183, 2176–2182 (2009).
Smith, K. G., Light, A., Nossal, G. J. & Tarlinton, D. M. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 16, 2996–3006 (1997).
Shlomchik, M. J. & Weisel, F. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 247, 52–63 (2012).
Purtha, W. E., Tedder, T. F., Johnson, S., Bhattacharya, D. & Diamond, M. S. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J. Exp. Med. 208, 2599–2606 (2011). This study provides evidence that memory B cells can respond to variants of the original pathogen that escape neutralization by antibodies produced by long-lived plasma cells.
Wrammert, J. et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208, 181–193 (2011).
Keating, R. et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nature Immunol. 14, 1266–1276 (2013).
Gao, F. et al. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell 158, 481–491 (2014).
van der Windt, G. J. W. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Chen, M. et al. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nature Med. 20, 503–510 (2014).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Forster, R., Emrich, T., Kremmer, E. & Lipp, M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 84, 830–840 (1994).
Rivino, L. et al. Chemokine receptor expression identifies pre-T helper TH1, pre-TH2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 200, 725–735 (2004).
Morita, R. et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2011).
Locci, M. et al. Human circulating PD-1+CXCR3−CXCR5+ memory TFH cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39, 758–769 (2013).
Bossaller, L. et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center TH cells. J. Immunol. 177, 4927–4932 (2006).
He, J. et al. Circulating precursor CCR7loPD-1hi CXCR5+ CD4+ T cells indicate TFH cell activity and promote antibody responses upon antigen re-exposure. Immunity 39, 770–781 (2013).
Tsai, L. M. & Yu, D. Follicular helper T-cell memory: establishing new frontiers during antibody response. Immunol. Cell Biol. 92, 57–63 (2014).
Boswell, K. L. et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 10, e1003853 (2014).
Bentebibel, S.-E. et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl Med. 5, 176ra32 (2013).
Acknowledgements
The authors thank Y. Takahashi and P. D. Burrows for critical reading of the manuscript, and T. Inoue for sharing unpublished data. This work was supported in part by grants provided by the Ministry of Education, Culture, Sports, Science, and Technology in Japan (to W.I. and T.K.); Japan Science and Technology Agency, Core Research for Evolutional Science and Technology (CREST; to T.K.); Secom Science and Technology Foundation (to T.K.); and RIKEN Special Postdoctoral Researchers Program (to K.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Isotype switching
-
A switch recombination in DNA that encodes the constant region of the immunoglobulin heavy chain, from IgM to any of IgG, IgA or IgE. The recombination occurs in repetitive DNA sequences (switch regions) that are located upstream of each constant region gene.
- Somatic hypermutation
-
A process in which point mutations are generated in the variable regions of immunoglobulin genes, thus creating a more specific repertoire when combined with selection. Some mutations might increase the affinity of the B cell receptor (BCR) for the specific antigen, but others might lead to a loss of antigen recognition by the BCR or to the generation of a self-reactive receptor.
- T follicular helper cells
-
(TFH cells). A distinct subset of antigen-activated CD4+ T cells expressing CXC-chemokine receptor 5 and B cell lymphoma 6. TFH cells are essential for germinal centre formation and regulate the activation and function of germinal centre B cells.
- B2 cells
-
The major and conventional B cell population in humans and mice. Marginal zone and follicular B cells belong to the B2 cell lineage and arise from bone marrow precursor cells.
- B1 cells
-
A self-renewing subset of mature B cells that predominates in the peritoneal and pleural cavities. B1 cells recognize self components, as well as common bacterial antigens, and are primarily responsible for the production of natural serum IgM.
- Fibroblastic reticular cells
-
(FRCs). The most abundant population of non-haematopoietic or stromal cells in T cell-rich areas of secondary lymphoid organs. FRCs facilitate interactions between T cells and dendritic cells, through the expression of cytokines and chemokines, such as interleukin-7, CC-chemokine ligand 19 (CCL19) and CCL21.
- Exhaustion
-
A term that was initially used to describe a state of T cell dysfunction that arises during many chronic infections and in cancer, and is typified by the increased expression of programmed cell death protein 1.
Rights and permissions
About this article
Cite this article
Kurosaki, T., Kometani, K. & Ise, W. Memory B cells. Nat Rev Immunol 15, 149–159 (2015). https://doi.org/10.1038/nri3802
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3802
This article is cited by
-
Abnormal frequency of the memory B cell subsets and plasmablasts in patients with congenital severe hemophilia A: correlation with “Inhibitor” formation
Blood Research (2024)
-
Memory B cells
Nature Reviews Immunology (2023)
-
Human memory B cells show plasticity and adopt multiple fates upon recall response to SARS-CoV-2
Nature Immunology (2023)
-
Beta variant COVID-19 protein booster vaccine elicits durable cross-neutralization against SARS-CoV-2 variants in non-human primates
Nature Communications (2023)
-
Antibody feedback regulates immune memory after SARS-CoV-2 mRNA vaccination
Nature (2023)