Abstract
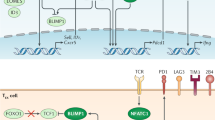
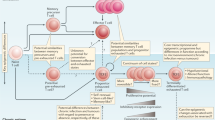
Chronic viral infections and malignant tumours induce T cells that have a reduced ability to secrete effector cytokines and have upregulated expression of the inhibitory receptor PD1 (programmed cell death protein 1). These features have so far been considered to mark terminally differentiated 'exhausted' T cells. However, several recent clinical and experimental observations indicate that phenotypically exhausted T cells can still mediate a crucial level of pathogen or tumour control. In this Opinion article, we propose that the exhausted phenotype results from a differentiation process in which T cells stably adjust their effector capacity to the needs of chronic infection. We argue that this phenotype is optimized to cause minimal tissue damage while still mediating a critical level of pathogen control. In contrast to the presently held view of functional exhaustion, this new concept better reflects the pathophysiology and clinical manifestations of persisting infections, and it provides a rationale for emerging therapies that enhance T cell activity in chronic infection and cancer by blocking inhibitory receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Badovinac, V. P., Haring, J. S. & Harty, J. T. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8+ T cell response to infection. Immunity 26, 827–841 (2007).
Miller, J. D. et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28, 710–722 (2008).
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature Rev. Immunol. 12, 749–761 (2012).
Arens, R. & Schoenberger, S. P. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol. Rev. 235, 190–205 (2010).
Zhang, N. & Bevan, M. J. CD8+ T cells: foot soldiers of the immune system. Immunity 35, 161–168 (2011).
Zehn, D., King, C., Bevan, M. J. & Palmer, E. TCR signaling requirements for activating T cells and for generating memory. Cell. Mol. Life Sci. 69, 1565–1575 (2012).
Jameson, S. C. & Masopust, D. Diversity in T cell memory: an embarrassment of riches. Immunity 31, 859–871 (2009).
Harari, A. et al. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211, 236–254 (2006).
Kuchroo, V. K., Anderson, A. C. & Petrovas, C. Coinhibitory receptors and CD8 T cell exhaustion in chronic infections. Curr. Opin. HIV AIDS 9, 439–445 (2014).
Chen, L. & Flies, D. B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature Rev. Immunol. 13, 227–242 (2013).
Gallimore, A. et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187, 1383–1393 (1998).
Moskophidis, D., Lechner, F., Pircher, H. & Zinkernagel, R. M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362, 758–761 (1993).
Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Blackburn, S. D. et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunol. 10, 29–37 (2009).
Wherry, E. J. T cell exhaustion. Nature Immunol. 12, 492–499 (2011).
Klenerman, P. & Hill, A. T cells and viral persistence: lessons from diverse infections. Nature Immunol. 6, 873–879 (2005).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nature Rev. Cancer 12, 252–264 (2012).
Kim, P. S. & Ahmed, R. Features of responding T cells in cancer and chronic infection. Curr. Opin. Immunol. 22, 223–230 (2010).
Crespo, J., Sun, H., Welling, T. H., Tian, Z. & Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 25, 214–221 (2013).
Wherry, E. J., Barber, D. L., Kaech, S. M., Blattman, J. N. & Ahmed, R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl Acad. Sci. USA 101, 16004–16009 (2004).
Virgin, H. W., Wherry, E. J. & Ahmed, R. Redefining chronic viral infection. Cell 138, 30–50 (2009).
Gairin, J. E., Mazarguil, H., Hudrisier, D. & Oldstone, M. B. Optimal lymphocytic choriomeningitis virus sequences restricted by H-2Db major histocompatibility complex class I molecules and presented to cytotoxic T lymphocytes. J. Virol. 69, 2297–2305 (1995).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Shin, H., Blackburn, S. D., Blattman, J. N. & Wherry, E. J. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204, 941–949 (2007).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Okazaki, T., Chikuma, S., Iwai, Y., Fagarasan, S. & Honjo, T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nature Immunol. 14, 1212–1218 (2013).
Price, D. A. et al. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity 21, 793–803 (2004).
Walker, B. & McMichael, A. The T-cell response to HIV. Cold Spring Harb. Perspect. Med. 2, a007054 (2012).
Hess, C. et al. HIV-1 specific CD8+ T cells with an effector phenotype and control of viral replication. Lancet 363, 863–866 (2004).
Ortiz, G. M. et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc. Natl Acad. Sci. USA 98, 13288–13293 (2001).
Fink, P. J. The biology of recent thymic emigrants. Annu. Rev. Immunol. 31, 31–50 (2013).
Douek, D. C. et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J. Immunol. 167, 6663–6668 (2001).
Freel, S. A., Saunders, K. O. & Tomaras, G. D. CD8+T-cell-mediated control of HIV-1 and SIV infection. Immunol. Res. 49, 135–146 (2011).
Schmitz, J. E. et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 (1999).
Jin, X. et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189, 991–998 (1999).
Hansen, S. G. et al. Immune clearance of highly pathogenic SIV infection. Nature 502, 100–104 (2013).
Mahnke, Y. D. et al. Human melanoma-specific CD8+ T-cells from metastases are capable of antigen-specific degranulation and cytolysis directly ex vivo. Oncoimmunology 1, 467–530 (2012).
Baitsch, L. et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Ahmadzadeh, M. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009).
Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. New Engl. J. Med. 348, 203–213 (2003).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nature Rev. Cancer 12, 298–306 (2012).
Blackburn, S. D. et al. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J. Virol. 84, 2078–2089 (2010).
Zelinskyy, G. et al. Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute friend retrovirus infection but are highly cytotoxic and control virus replication. J. Immunol. 187, 3730–3737 (2011).
Larsen, M. et al. Exhausted cytotoxic control of Epstein-Barr virus in human lupus. PLoS Pathog. 7, e1002328 (2011).
Sakhdari, A. et al. Tim-3 negatively regulates cytotoxicity in exhausted CD8+ T cells in HIV infection. PLoS ONE 7, e40146 (2012).
Fourcade, J. et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 207, 2175–2186 (2010).
Jin, H. T. et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl Acad. Sci. USA 107, 14733–14738 (2010).
Nakamoto, N. et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 5, e1000313 (2009).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Woo, S. R. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012).
Blackburn, S. D., Shin, H., Freeman, G. J. & Wherry, E. J. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl Acad. Sci. USA 105, 15016–15021 (2008).
Matloubian, M., Concepcion, R. J. & Ahmed, R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68, 8056–8063 (1994).
Snyder, C. M. et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29, 650–659 (2008).
Ibegbu, C. C. et al. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J. Immunol. 174, 6088–6094 (2005).
Hislop, A. D., Taylor, G. S., Sauce, D. & Rickinson, A. B. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25, 587–617 (2007).
Freeman, M. L., Burkum, C. E., Jensen, M. K., Woodland, D. L. & Blackman, M. A. γ-Herpesvirus reactivation differentially stimulates epitope-specific CD8 T cell responses. J. Immunol. 188, 3812–3819 (2012).
Appay, V. et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Med. 8, 379–385 (2002).
Hertoghs, K. M. et al. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J. Clin. Invest. 120, 4077–4090 (2010).
Lichterfeld, M. et al. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J. Virol. 81, 4199–4214 (2007).
Vigano, S. et al. Rapid perturbation in viremia levels drives increases in functional avidity of HIV-specific CD8 T cells. PLoS Pathog. 9, e1003423 (2013).
Utzschneider, D. T. et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nature Immunol. 14, 603–610 (2013).
Youngblood, B. et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity 35, 400–412 (2011).
Youngblood, B. et al. Cutting edge: Prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J. Immunol. 191, 540–544 (2013).
Kasprowicz, V. et al. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J. Virol. 82, 3154–3160 (2008).
Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Gerlach, C. et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science 340, 635–639 (2013).
Buchholz, V. R. et al. Disparate individual fates compose robust CD8+ T cell immunity. Science 340, 630–635 (2013).
Williams, M. A. & Bevan, M. J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25, 171–192 (2007).
Paley, M. A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012).
Sun, J. C. & Bevan, M. J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300, 339–342 (2003).
Waggoner, S. N., Cornberg, M., Selin, L. K. & Welsh, R. M. Natural killer cells act as rheostats modulating antiviral T cells. Nature 481, 394–398 (2012).
Angelosanto, J. M., Blackburn, S. D., Crawford, A. & Wherry, E. J. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 86, 8161–8170 (2012).
Doering, T. A. et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144 (2012).
Cornberg, M. et al. Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Frontiers Immunol. 4, 475 (2013).
Doedens, A. L. et al. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nature Immunol. 14, 1173–1182 (2013).
Frebel, H. et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209, 2485–2499 (2012).
Mueller, S. N. et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J. Clin. Invest. 120, 2508–2515 (2010).
Hirano, F. et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 65, 1089–1096 (2005).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl. J. Med. 366, 2443–2454 (2012).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Wakim, L. M., Waithman, J., van Rooijen, N., Heath, W. R. & Carbone, F. R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunol. 10, 524–530 (2009).
Jiang, X. et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231 (2012).
Mueller, S. N., Gebhardt, T., Carbone, F. R. & Heath, W. R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161 (2013).
Wakim, L. M. et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol. 189, 3462–3471 (2012).
Zhu, J. et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 497, 494–497 (2013).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl. J. Med. 363, 711–723 (2010).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl. J. Med. 366, 2455–2465 (2012).
Ribas, A. Tumor immunotherapy directed at PD-1. New Engl. J. Med. 366, 2517–2519 (2012).
Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. New Engl. J. Med. 369, 134–144 (2013).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. New Engl. J. Med. 369, 122–133 (2013).
Riley, J. L. Combination checkpoint blockade — taking melanoma immunotherapy to the next level. New Engl. J. Med. 369, 187–189 (2013).
Korman, A. J., Peggs, K. S. & Allison, J. P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 90, 297–339 (2006).
Gardiner, D. et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE 8, e63818 (2013).
Fuller, M. J. et al. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1). Proc. Natl Acad. Sci. USA 110, 15001–15006 (2013).
Velu, V. et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458, 206–210 (2009).
Minter, S., Willner, I. & Shirai, K. Ipilimumab-induced hepatitis C viral suppression. J. Clin. Oncol. 31, e307–e308 (2013).
Acknowledgements
D.E.S., C.M., P.R. and D.Z. are supported by a Swiss National Science Sinergia grant (FNS CRSII3_141879).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
PowerPoint slides
Rights and permissions
About this article
Cite this article
Speiser, D., Utzschneider, D., Oberle, S. et al. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion?. Nat Rev Immunol 14, 768–774 (2014). https://doi.org/10.1038/nri3740
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3740
This article is cited by
-
Intimate communications within the tumor microenvironment: stromal factors function as an orchestra
Journal of Biomedical Science (2023)
-
Tyrosine kinase signaling-independent MET-targeting with CAR-T cells
Journal of Translational Medicine (2023)
-
Exhausted phenotype of circulating CD8+ T cell subsets in hepatitis B virus carriers
BMC Immunology (2022)
-
Blocking siglec-10hi tumor-associated macrophages improves anti-tumor immunity and enhances immunotherapy for hepatocellular carcinoma
Experimental Hematology & Oncology (2021)
-
Transcriptional regulatory network for the establishment of CD8+ T cell exhaustion
Experimental & Molecular Medicine (2021)