Key Points
-
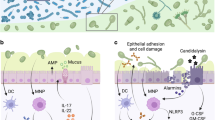
The fungal microbiota, or 'mycobiota', is an understudied component of the microflora that is found on all mucosal surfaces and on the skin.
-
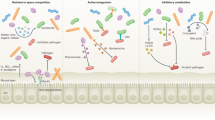
Like other microorganisms, fungi interact with the immune system at these surfaces in ways that are important both for host defence and for regulating the immune system.
-
Investigators who study the mycobiota face both biological and bioinformatic challenges.
-
The study of human genetic disorders and genetic polymorphisms teaches us about the mechanisms by which commensal and pathogenic fungi interact with the immune system.
Abstract
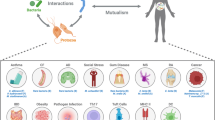
The body is host to a wide variety of microbial communities from which the immune system protects us and that are important for the normal development of the immune system and for the maintenance of healthy tissues and physiological processes. Investigators have mostly focused on the bacterial members of these communities, but fungi are increasingly being recognized to have a role in defining these communities and to interact with immune cells. In this Review, we discuss what is currently known about the makeup of fungal communities in the body and the features of the immune system that are particularly important for interacting with fungi at these sites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Clemente, J. C., Ursell, L. K., Parfrey, L. W. & Knight, R. The impact of the gut microbiota on human health: an integrative view. Cell 148, 1258–1270 (2012).
Erturk-Hasdemir, D. & Kasper, D. L. Resident commensals shaping immunity. Curr. Opin. Immunol. 25, 450–455 (2013).
Tremaroli, V. & Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
Bull-Otterson, L. et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 8, e53028 (2013).
Sonnenberg, G. F. et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336, 1321–1325 (2012).
Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487, 104–108 (2012).
Nilsson, R. H. et al. Taxonomic reliability of DNA sequences in public sequence databases: a fungal perspective. PLoS ONE 1, e59 (2006).
Hube, B. From commensal to pathogen: stage- and tissue-specific gene expression of Candida albicans. Curr. Opin. Microbiol. 7, 336–341 (2004).
Scupham, A. J. et al. Abundant and diverse fungal microbiota in the murine intestine. Appl. Environ. Microbiol. 72, 793–801 (2006). This was the first culture-independent large-scale analysis of the distribution of fungal rRNA genes in the mammalian intestine and it shows the rich fungal diversity that is present in the mouse gut.
Iliev, I. D. et al. Interactions between commensal fungi and the C-Type lectin receptor dectin-1 influence colitis. Science 336, 1314–1317 (2012).
Dollive, S. et al. Fungi of the murine gut: episodic variation and proliferation during antibiotic treatment. PLoS ONE 8, e71806 (2013).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2013).
Hoffmann, C. et al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS ONE 8, e66019 (2013). This study demonstrates an effect of long-term diet in determining the structure of the human gut microbiome and shows that there are correlations between bacteria, fungi and archaea.
Odds, F. C. et al. Candida albicans strain maintenance, replacement, and microvariation demonstrated by multilocus sequence typing. J. Clin. Microbiol. 44, 3647–3658 (2006).
Standaert-Vitse, A. et al. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn's disease. Gastroenterology 130, 1764–1775 (2006).
Ott, S. J. et al. Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scand. J. Gastroenterol. 43, 831–841 (2008). This study provides the first evidence for increased fungal diversity and an alteration of intestinal mycobiota profiles in patients with IBD.
Scanlan, P. D. & Marchesi, J. R. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. ISME J. 2, 1183–1193 (2008).
Standaert-Vitse, A. et al. Candida albicans colonization and ASCA in familial Crohn's disease. Am. J. Gastroenterol. 104, 1745–1753 (2009).
Angebault, C. et al. Candida albicans is not always the preferential yeast colonizing humans: a study in Wayampi Amerindians. J. Infect. Dis. 208, 1705–1716 (2013).
Savage, D. C., Dubos, R. & Schaedler, R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J. Exp. Med. 127, 67–76 (1968).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Agans, R. et al. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol. Ecol. 77, 404–412 (2011).
Naglik, J. R., Fidel, P. L. Jr & Odds, F. C. Animal models of mucosal Candida infection. FEMS Microbiol. Lett. 283, 129–139 (2008).
Noverr, M. C., Falkowski, N. R., McDonald, R. A., McKenzie, A. N. & Huffnagle, G. B. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect. Immun. 73, 30–38 (2005).
Mason, K. L. et al. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 80, 3371–3380 (2012).
Samonis, G. et al. Prospective evaluation of effects of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans. Antimicrob. Agents Chemother. 37, 51–53 (1993).
Mulligan, M. E., Citron, D. M., McNamara, B. T. & Finegold, S. M. Impact of cefoperazone therapy on fecal flora. Antimicrob. Agents Chemother. 22, 226–230 (1982).
Karabinis, A. et al. Risk factors for candidemia in cancer patients: a case-control study. J. Clin. Microbiol. 26, 429–432 (1988).
Richardson, M. D. Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 56, i5–i11 (2005).
Zaoutis, T. E. et al. Risk factors and predictors for candidemia in pediatric intensive care unit patients: implications for prevention. Clin. Infect. Dis. 51, e38–45 (2010).
Erb-Downward, J. R., Falkowski, N. R., Mason, K. L., Muraglia, R. & Huffnagle, G. B. Modulation of post-antibiotic bacterial community reassembly and host response by Candida albicans. Sci. Rep. 3, 2191 (2013). This study shows that during antibiotic-induced dysbiosis, the exogenous addition of C. albicans will lead to overgrowth and will influence the composition of the bacterial microbiota.
Brown, G. D. Innate antifungal immunity: the key role of phagocytes. Annu. Rev. Immunol. 29, 1–21 (2011).
Romani, L. Immunity to fungal infections. Nature Rev. Immunol. 11, 275–288 (2011).
Khor, B., Gardet, A. & Xavier, R. J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Beaudoin, M. et al. Deep resequencing of GWAS loci identifies rare variants in CARD9, IL23R and RNF186 that are associated with ulcerative colitis. PLoS Genet. 9, e1003723 (2013).
Glocker, E. O. et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361, 1727–1735 (2009). This was the first paper to demonstrate that genetic impairment of CARD9 leaves individuals highly susceptible to CMC.
Sokol, H. et al. Card9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice. Gastroenterology 145, 591–601 (2013).
De Luca, A. et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal. Immunol. 3, 361–373 (2010). This study demonstrates a protective role of IL-22 in the gastrointestinal mucosa during Candida infection.
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Noverr, M. C., Noggle, R. M., Toews, G. B. & Huffnagle, G. B. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 72, 4996–5003 (2004).
Noverr, M. C., Phare, S. M., Toews, G. B., Coffey, M. J. & Huffnagle, G. B. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 69, 2957–2963 (2001).
Erb-Downward, J. R. & Noverr, M. C. Characterization of prostaglandin E2 production by Candida albicans. Infect. Immun. 75, 3498–3505 (2007).
Noverr, M. C., Toews, G. B. & Huffnagle, G. B. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect. Immun. 70, 400–402 (2002).
Kim, Y. G. et al. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE2 . Cell Host Microbe 15, 95–102 (2014).
van der Velden, W. J. et al. Role of the mycobiome in human acute graft-versus-host disease. Biol. Blood Marrow Transplant. 19, 329–332 (2013).
Ghannoum, M. A. et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 6, e1000713 (2010).
Dupuy, A. K. et al. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: discovery of Malassezia as a prominent commensal. PLoS ONE 9, e90899 (2014).
Smeekens, S. P., van de Veerdonk, F. L., Kullberg, B. J. & Netea, M. G. Genetic susceptibility to Candida infections. EMBO Mol. Med. 5, 805–813 (2013).
van de Veerdonk, F. L. et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 365, 54–61 (2011).
Takezaki, S. et al. Chronic mucocutaneous candidiasis caused by a gain-of-function mutation in the STAT1 DNA- binding domain. J. Immunol. 189, 1521–1526 (2012).
Smeekens, S. P. et al. STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS ONE 6, e29248 (2011).
Liu, L. et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 208, 1635–1648 (2011).
Puel, A. et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332, 65–68 (2011). This study demonstrates that genetic deficiencies in the IL-17 signalling pathway predispose individuals to CMC, which provides a link between IL-17 and mucosal antifungal immunity in humans.
Boisson, B. et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 39, 676–686 (2013).
Puel, A. et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207, 291–297 (2010).
Conti, H. R. et al. TH17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206, 299–311 (2009). This study shows a crucial role for IL-17 signalling in controlling Candida in the oral mucosa.
Gladiator, A., Wangler, N., Trautwein-Weidner, K. & LeibundGut-Landmann, S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J. Immunol. 190, 521–525 (2013).
Lanternier, F. et al. Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 369, 1704–1714 (2013).
Drewniak, A. et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 121, 2385–2392 (2013).
Bishu, S. et al. The adaptor CARD9 is required for adaptive but not innate immunity to oral mucosal Candida albicans infections. Infect. Immun. 82, 1173–1180 (2013).
Hise, A. G. et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009). Using a mouse model of oral infection, this study demonstrates that CLEC7A, NLRP3 and TLR2 are important for controlling C. albicans.
Ferwerda, B. et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361, 1760–1767 (2009). This was the first paper to demonstrate that genetic impairment of CLEC7A in humans impairs host defence against Candida infection.
Robinson, M. J. et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for TH17 responses to fungal infection. J. Exp. Med. 206, 2037–2051 (2009).
Grice, E. A. et al. Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192 (2009).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009).
Findley, K. et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–370 (2013). This study is the most comprehensive culture- independent evaluation to date of the communities of fungi that are associated with the human skin and highlights the dominance of Malassezia species.
Tagami, H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 30, 413–434 (2008).
Grice, E. A. & Segre, J. A. The skin microbiome. Nature Rev. Microbiol. 9, 244–253 (2011).
Roth, R. R. & James, W. D. Microbial ecology of the skin. Annu. Rev. Microbiol. 42, 441–464 (1988).
Paulino, L. C., Tseng, C. H., Strober, B. E. & Blaser, M. J. Molecular analysis of fungal microbiota in samples from healthy human skin and psoriatic lesions. J. Clin. Microbiol. 44, 2933–2941 (2006).
Zhang, E. et al. Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiol. Immunol. 55, 625–632 (2011).
Oh, J. et al. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res. 23, 2103–2114 (2013).
Smeekens, S. P. et al. Skin microbiome imbalance in patients with STAT1/STAT3 defects impairs innate host defense responses. J. Innate Immun. 6, 253–262 (2014).
Kagami, S., Rizzo, H. L., Kurtz, S. E., Miller, L. S. & Blauvelt, A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J. Immunol. 185, 5453–5462 (2010).
Drell, T. et al. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS ONE 8, e54379 (2013).
Zheng, N. N., Guo, X. C., Lv, W., Chen, X. X. & Feng, G. F. Characterization of the vaginal fungal flora in pregnant diabetic women by 18S rRNA sequencing. Eur. J. Clin. Microbiol. Infect. Dis. 32, 1031–1040 (2013).
Guo, R. et al. Increased diversity of fungal flora in the vagina of patients with recurrent vaginal candidiasis and allergic rhinitis. Microb. Ecol. 64, 918–927 (2012).
Boris, S. & Barbes, C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2, 543–546 (2000).
Boris, S., Suarez, J. E., Vazquez, F. & Barbes, C. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 66, 1985–1989 (1998).
Kohler, G. A., Assefa, S. & Reid, G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect. Dis. Obstet. Gynecol. 2012, 636474 (2012).
De Luca, A. et al. IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis. PLoS Pathog. 9, e1003486 (2013).
Lev-Sagie, A. et al. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am. J. Obstet. Gynecol. 200, 303. e1–6 (2009).
Tomalka, J. et al. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 7, e1002379 (2011).
Wojitani, M. D., de Aguiar, L. M., Baracat, E. C. & Linhares, I. M. Association between mannose-binding lectin and interleukin-1 receptor antagonist gene polymorphisms and recurrent vulvovaginal candidiasis. Arch. Gynecol. Obstet. 285, 149–153 (2012).
Babula, O., Lazdane, G., Kroica, J., Ledger, W. J. & Witkin, S. S. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin. Infect. Dis. 37, 733–737 (2003).
Giraldo, P. C. et al. Mannose-binding lectin gene polymorphism, vulvovaginal candidiasis, and bacterial vaginosis. Obstet. Gynecol. 109, 1123–1128 (2007).
Crosdale, D. J., Poulton, K. V., Ollier, W. E., Thomson, W. & Denning, D. W. Mannose-binding lectin gene polymorphisms as a susceptibility factor for chronic necrotizing pulmonary aspergillosis. J. Infect. Dis. 184, 653–656 (2001).
van Woerden, H. C. et al. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infect. Dis. 13, 69 (2013).
Pihet, M. et al. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis — a review. Med. Mycol. 47, 387–397 (2009).
Delhaes, L. et al. The airway microbiota in cystic fibrosis: a complex fungal and bacterial community — implications for therapeutic management. PLoS ONE 7, e36313 (2012).
Dagenais, T. R. & Keller, N. P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 22, 447–465 (2009).
Agarwal, R. et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin. Exp. Allergy 43, 850–873 (2013).
Hohl, T. M. et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific β-glucan display. PLoS Pathog. 1, e30 (2005).
Gersuk, G. M., Underhill, D. M., Zhu, L. & Marr, K. A. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 176, 3717–3724 (2006).
Carrion Sde, J. et al. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J. Immunol. 191, 2581–2588 (2013).
Faro-Trindade, I. et al. Characterisation of innate fungal recognition in the lung. PLoS ONE 7, e35675 (2012).
Grimm, M. J. et al. Monocyte- and macrophage-targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice. J. Immunol. 190, 4175–4184 (2013).
Grimm, M. J. et al. Role of NADPH oxidase in host defense against aspergillosis. Med. Mycol. 49 (Suppl. 1), 144–149 (2011).
Lass-Florl, C., Roilides, E., Loffler, J., Wilflingseder, D. & Romani, L. Minireview: host defence in invasive aspergillosis. Mycoses 56, 403–413 (2013).
Rivera, A. et al. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 208, 369–381 (2011).
Gessner, M. A. et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect. Immun. 80, 410–417 (2012).
Taylor, P. R. et al. Activation of neutrophils by autocrine IL-17A–IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nature Immunol. 15, 143–151 (2014). This study shows that neutrophils are an important source of IL-17 in response to fungi such as Aspergillus.
Lilly, L. M. et al. The β-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J. Immunol. 189, 3653–3660 (2012).
Mintz-Cole, R. A. et al. Dectin-1 and IL-17A suppress murine asthma induced by Aspergillus versicolor but not Cladosporium cladosporioides due to differences in β-glucan surface exposure. J. Immunol. 189, 3609–3617 (2012).
Bi, L. et al. CARD9 mediates dectin-2-induced IκBα kinase ubiquitination leading to activation of NF-κB in response to stimulation by the hyphal form of Candida albicans. J. Biol. Chem. 285, 25969–25977 (2010).
Saijo, S. et al. Dectin-2 recognition of α-mannans and induction of TH17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691 (2010).
Moyes, D. L. et al. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE 6, e26580 (2011).
Cheng, S. C. et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J. Leukoc. Biol. 90, 357–366 (2011).
Gantner, B. N., Simmons, R. M. & Underhill, D. M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24, 1277–1286 (2005).
Wheeler, R. T., Kombe, D., Agarwala, S. D. & Fink, G. R. Dynamic, morphotype-specific Candida albicans β-glucan exposure during infection and drug treatment. PLoS Pathog. 4, e1000227 (2008).
Pande, K., Chen, C. & Noble, S. M. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nature Genet. 45, 1088–1091 (2013).
Zhang, Q. et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361, 2046–2055 (2009).
Engelhardt, K. R. et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J. Allergy Clin. Immunol. 124, 1289–1302 (2009).
Holland, S. M. et al. STAT3 mutations in the hyper IgE syndrome. N. Engl. J. Med. 357, 1608–1619 (2007).
Milner, J. D. et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 (2008).
Freeman, A. F. et al. Causes of death in hyper-IgE syndrome. J. Allergy Clin. Immunol. 119, 1234–1240 (2007).
Minegishi, Y. et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448, 1058–1062 (2007).
Plantinga, T. S. et al. Early stop polymorphism in human DECTIN-1 is associated with increased Candida colonization in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 49, 724–732 (2009).
Ouederni, M. et al. Clinical features of Candidiasis in patients with inherited interleukin 12 receptor β1 deficiency. Clin. Infect. Dis. 58, 204–213 (2014).
Acknowledgements
The authors' work relating to this manuscript was funded by the US National Institutes of Health (grant DK098310 to I.D.I. and DK093426 to D.M.U.), as well as the Crohn's and Colitis Foundation of America.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Inflammatory bowel disease
-
(IBD). A group of chronic inflammatory conditions that affect the large and small intestine. The major types are Crohn's disease and ulcerative colitis.
- Dysbiosis
-
A term that was originally coined by the 1908 Nobel laureate Eli Metchnikoff to refer to pathogenic alterations of the bacterial microflora in the gut. Now used more generally to refer to any microbial imbalance in or on the body at sites including the gastrointestinal tract, the skin and exposed mucosal surfaces such as the lungs, vagina or mouth.
- Shotgun sequencing
-
A sequencing approach in which a complex pool of DNA is broken up into random small segments that are sequenced en masse. Computational tools are then used to reassemble and characterize the DNA fragments.
- Uncultured
-
A term that is used in mycobiome sequencing studies to refer to sequences that are identified in the National Center for Biotechnology Information GenBank database as fungal but that are currently of uncharacterized origin.
- Candida
-
A genus of yeasts that includes common species such as Candida albicans, C. tropicalis, C. glabrata, C. parapsilosis and C. krusei. Candida species are normal inhabitants of the skin and mucous membranes and primarily cause disease in immunocompromised individuals. Candidiasis (disease caused by Candida) of the mouth or throat is called thrush or oropharyngeal candidiasis.
- Anti-Saccharomyces cerevisiae antibodies
-
(ASCAs). Antibodies that are commonly found in serum from patients with inflammatory bowel disease. These antibodies are more common in individuals with Crohn's disease than in patients with ulcerative colitis. ASCAs cross-react with mannans from the cell walls of many fungi (including Candida species), which suggests that the name might be misleading.
- Caspase recruitment domain-containing protein 9
-
(CARD9). A signalling adaptor molecule that functions downstream of many immunoreceptor tyrosine- based activation motif (ITAM) receptors that are present in phagocytes, including macrophages and dendritic cells. CARD9 associates with B cell lymphoma 10 (BCL-10) and the paracaspase MALT1 to facilitate signalling through nuclear factor-κB and to promote acute inflammatory responses and the initiation of adaptive immunity.
- T helper 1 (TH1) cell-mediated immunity
-
An immune response that is characterized by T cells that produce IFNγ. This is generally associated with effective host defence against intracellular bacteria and protozoa.
- Malassezia
-
A genus of basidiomycetous fungi that includes species such as Malassezia dermatis, M. furfur and M. restricta. These yeasts are specifically adapted for growth on mammalian skin and they are associated with conditions such as dandruff, atopic eczema and dermatitis, pityriasis versicolor, seborrheic dermatitis and folliculitis.
- Chronic mucocutaneous candidiasis
-
(CMC). A condition that is characterized by recurrent Candida infections of the mouth, skin and other mucosal surfaces.
- Onychomycosis
-
A fungal infection of the toenails or fingernails. These infections are most commonly caused by dermatophytes (Microsporum, Epidermophyton and Trichophyton) but can also be caused by Candida species and non-dermatophytic moulds.
- C-type lectin receptor
-
(CLR). A member of a large family of receptors that bind to carbohydrates, typically in a calcium-dependent manner. Here, we use the term to refer to the set of CLRs that act as 'pattern recognition receptors' in the detection of microbial threats and that activate immune responses. The CLR family includes membrane receptors, such as CLEC6A, CLEC7A and the mannose receptor, and also soluble receptors, such as mannose-binding lectin (MBL). The binding activity of these receptors is mediated by conserved carbohydrate-recognition domains.
- a–α mating type
-
This refers to two haploid sexual forms of yeast. The a-type yeast cells secret an 'a-factor', which is a pheromone that attracts the α-mating type, which, in turn, secretes 'α-factor'. The a-type cells respond to the α-factor by growing a projection (shmoo) towards the α-cells. Haploid cells respond only to a pheromone of the opposite cell type, which allows for mating to only occur between a-type and α-type cells.
Rights and permissions
About this article
Cite this article
Underhill, D., Iliev, I. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol 14, 405–416 (2014). https://doi.org/10.1038/nri3684
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3684
This article is cited by
-
Fungal coexistence in the skin mycobiome: a study involving Malassezia, Candida, and Rhodotorula
AMB Express (2024)
-
Gut and bladder fermentation syndromes: a narrative review
BMC Medicine (2024)
-
VESPA: an optimized protocol for accurate metabarcoding-based characterization of vertebrate eukaryotic endosymbiont and parasite assemblages
Nature Communications (2024)
-
Discovery of antifungal secondary metabolites from an intestinal fungus Fusarium sp.
The Journal of Antibiotics (2024)
-
Unravelling the temporal and spatial variation of fungal phylotypes from embryo to adult stages in Atlantic salmon
Scientific Reports (2024)