Key Points
-
Mast cells and basophils are phenotypically and functionally related cell types that promote protective immunity to helminths, but that can also cause pathology during allergic inflammation.
-
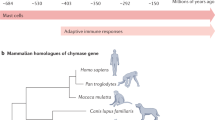
Mast cells and basophils might have evolved from a common precursor cell, such as the mast cell/basophil-like (MCBL) cell or the test cell, which were identified in the urochordate Styela plicata. A basophil/mast cell progenitor (BMCP) cell population was isolated from the spleen of adult mice, and the timed expression of the transcription factors GATA-binding protein 2 and CCAAT/enhancer-binding protein-α in these progenitor cells might determine whether they eventually mature into basophils or mast cells.
-
New mouse models have been developed in which mast cells or basophils can be constitutively or conditionally depleted. These models allow more specific targeting of mast cells and basophils, compared with the Kit-mutant mouse strains and depleting antibody strategies which were previously used to assess mast cell and basophil functions in vivo. Therefore, these new mouse models will help to further clarify the true biological roles of basophils and mast cells.
-
Although mast cells and basophils can express interleukin-4, CD40 ligand and low levels of MHC class II under certain conditions, they seem to have no major role in driving the induction of adaptive type 2 immune responses.
-
Mast cells and basophils cooperate to provide protection during secondary infestation with ticks. In addition, both cell types contribute, to various degrees, to protective immunity during gastrointestinal helminth infections in mice.
-
IgE-mediated anaphylaxis is strictly mast cell-dependent, whereas IgG1-mediated anaphylaxis in mice results from the activation of monocytes, neutrophils or basophils. Basophils are recruited to the skin in a mouse model of atopic dermatitis and they induce IgE-mediated chronic allergic inflammation of the skin in the absence of mast cells.
Abstract
Mast cells and basophils are potent effector cells of the innate immune system, and they have both beneficial and detrimental functions for the host. They are mainly implicated in pro-inflammatory responses to allergens but can also contribute to protection against pathogens. Although both cell types were identified more than 130 years ago by Paul Ehrlich, their in vivo functions remain poorly understood. The precursor cell populations that give rise to mast cells and basophils have recently been characterized and isolated. Furthermore, new genetically modified mouse strains have been developed, which enable more specific targeting of mast cells and basophils. Such advances offer new opportunities to uncover the true in vivo activities of these cells and to revisit their previously proposed effector functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Migalovich-Sheikhet, H., Friedman, S., Mankuta, D. & Levi-Schaffer, F. Novel identified receptors on mast cells. Front. Immunol. 3, 238 (2012).
Schneider, E., Thieblemont, N., De Moraes, M. L. & Dy, M. Basophils: new players in the cytokine network. Eur. Cytokine Netw 21, 142–153 (2010).
Gilfillan, A. M. & Beaven, M. A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 31, 475–529 (2011).
Karasuyama, H., Mukai, K., Obata, K., Tsujimura, Y. & Wada, T. Nonredundant roles of basophils in immunity. Annu. Rev. Immunol. 29, 45–69 (2011).
Lichtenstein, L. M. & Bochner, B. S. The role of basophils in asthma. Ann. NY Acad. Sci. 629, 48–61 (1991).
Beaven, M. A. Our perception of the mast cell from Paul Ehrlich to now. Eur. J. Immunol. 39, 11–25 (2009).
Abraham, S. N. & St John, A. L. Mast cell-orchestrated immunity to pathogens. Nature Rev. Immunol. 10, 440–452 (2010).
Feger, F., Varadaradjalou, S., Gao, Z., Abraham, S. N. & Arock, M. The role of mast cells in host defense and their subversion by bacterial pathogens. Trends Immunol. 23, 151–158 (2002).
Galli, S. J. et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 23, 749–786 (2005).
Galli, S. J., Nakae, S. & Tsai, M. Mast cells in the development of adaptive immune responses. Nature Immunol. 6, 135–142 (2005).
Falcone, F. H., Zillikens, D. & Gibbs, B. F. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp. Dermatol. 15, 855–864 (2006).
Siracusa, M. C., Comeau, M. R. & Artis, D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann. NY Acad. Sci. 1217, 166–177 (2011).
Crivellato, E., Beltrami, C., Mallardi, F. & Ribatti, D. Paul Ehrlich's doctoral thesis: a milestone in the study of mast cells. Br. J. Haematol. 123, 19–21 (2003).
de Barros, C. M. et al. The Hemolymph of the ascidian Styela plicata (Chordata-Tunicata) contains heparin inside basophil-like cells and a unique sulfated galactoglucan in the plasma. J. Biol. Chem. 282, 1615–1626 (2007).
Cavalcante, M. C. et al. Colocalization of heparin and histamine in the intracellular granules of test cells from the invertebrate Styela plicata (Chordata-Tunicata). J. Struct. Biol. 137, 313–321 (2002).
Dobson, J. T. et al. Carboxypeptidase A5 identifies a novel mast cell lineage in the zebrafish providing new insight into mast cell fate determination. Blood 112, 2969–2972 (2008).
Da'as, S. et al. Zebrafish mast cells possess an FcεRI-like receptor and participate in innate and adaptive immune responses. Dev. Comparative Immunol. 35, 125–134 (2011).
Mulero, I., Sepulcre, M. P., Meseguer, J., Garcia-Ayala, A. & Mulero, V. Histamine is stored in mast cells of most evolutionarily advanced fish and regulates the fish inflammatory response. Proc. Natl Acad. Sci. USA 104, 19434–19439 (2007).
Mead, K. F., Borysenko, M. & Findlay, S. R. Naturally abundant basophils in the snapping turtle, Chelydra serpentina, possess cytophilic surface antibody with reaginic function. J. Immunol. 130, 334–340 (1983).
Rodewald, H. R., Dessing, M., Dvorak, A. M. & Galli, S. J. Identification of a committed precursor for the mast cell lineage. Science 271, 818–822 (1996).
Ohnmacht, C. & Voehringer, D. Basophil effector function and homeostasis during helminth infection. Blood 113, 2816–2825 (2009).
Iwasaki, H. et al. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev. 20, 3010–3021 (2006).
Chen, C. C., Grimbaldeston, M. A., Tsai, M., Weissman, I. L. & Galli, S. J. Identification of mast cell progenitors in adult mice. Proc. Natl Acad. Sci. USA 102, 11408–11413 (2005).
Franco, C. B., Chen, C. C., Drukker, M., Weissman, I. L. & Galli, S. J. Distinguishing mast cell and granulocyte differentiation at the single-cell level. Cell Stem Cell 6, 361–368 (2010).
Arinobu, Y. et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc. Natl Acad. Sci. USA 102, 18105–18110 (2005). Identification of a common BMCP in the spleen of adult mice. The timed expression of GATA2 and C/EBPα can direct the final maturation of BMCPs to basophils or mast cells.
Ohnmacht, C. et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33, 364–374 (2010).
Ohmori, K. et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J. Immunol. 182, 2835–2841 (2009).
Siracusa, M. C. et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477, 229–233 (2011).
Mukai, K. et al. Critical role of P1-Runx1 in mouse basophil development. Blood 120, 76–85 (2012).
Jamur, M. C. et al. Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood 105, 4282–4289 (2005).
Martin, D. I., Zon, L. I., Mutter, G. & Orkin, S. H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature 344, 444–447 (1990).
Buhring, H. J., Streble, A. & Valent, P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int. Arch. Allergy Immunol. 133, 317–329 (2004).
Arock, M., Schneider, E., Boissan, M., Tricottet, V. & Dy, M. Differentiation of human basophils: an overview of recent advances and pending questions. J. Leukoc. Biol. 71, 557–564 (2002).
Kitamura, Y., Hatanaka, K., Murakami, M. & Shibata, H. Presence of mast cell precursors in peripheral blood of mice demonstrated by parabiosis. Blood 53, 1085–1088 (1979).
Gurish, M. F. & Austen, K. F. Developmental origin and functional specialization of mast cell subsets. Immunity 37, 25–33 (2012).
Welle, M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J. Leukoc. Biol. 61, 233–245 (1997).
Li, L. et al. Identification of basophilic cells that express mast cell granule proteases in the peripheral blood of asthma, allergy, and drug-reactive patients. J. Immunol. 161, 5079–5086 (1998).
Kim, S. et al. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J. Immunol. 184, 1143–1147 (2010).
Kiernan, J. A. Production and life span of cutaneous mast cells in young rats. J. Anat. 128, 225–238 (1979).
Lantz, C. S. et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 392, 90–93 (1998).
Didichenko, S. A., Spiegl, N., Brunner, T. & Dahinden, C. A. IL-3 induces a Pim1-dependent antiapoptotic pathway in primary human basophils. Blood 112, 3949–3958 (2008).
Shelburne, C. P. et al. Stat5 expression is critical for mast cell development and survival. Blood 102, 1290–1297 (2003).
Lilla, J. N. et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood 118, 6930–6938 (2011).
Asai, K. et al. Regulation of mast cell survival by IgE. Immunity 14, 791–800 (2001).
Kashiwakura, J. et al. Most highly cytokinergic IgEs have polyreactivity to autoantigens. Allergy Asthma Immunol. Res. 4, 332–340 (2012).
Hill, D. A. et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nature Med. 18, 538–546 (2012).
Hida, S. et al. Fc receptor γ-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nature Immunol. 10, 214–222 (2009).
Herbst, T. et al. Antibodies and IL-3 support helminth-induced basophil expansion. Proc. Natl Acad. Sci. USA 109, 14954–14959 (2012).
Reber, L. L., Marichal, T. & Galli, S. J. New models for analyzing mast cell functions in vivo. Trends Immunol. 33, 613–625 (2012).
Rodewald, H. R. & Feyerabend, T. B. Widespread immunological functions of mast cells: fact or fiction? Immunity 37, 13–24 (2012). This review discusses controversial findings obtained with Kit -mutant mice and newly developed mast cell-deficient mouse strains regarding the role of mast cells in various murine disease models.
Jonsson, F. & Daeron, M. Mast cells and company. Front. Immunol. 3, 16 (2012).
Dudeck, A. et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34, 973–984 (2011).
Sawaguchi, M. et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J. Immunol. 188, 1809–1818 (2012).
Feyerabend, T. B. et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity 35, 832–844 (2011).
Schmidt-Supprian, M. & Rajewsky, K. Vagaries of conditional gene targeting. Nature Immunol. 8, 665–668 (2007). This review points out that high expression levels of Cre can lead to Cre toxicity in certain cell types owing to recognition of cryptic loxP sites in the genome.
Kojima, T. et al. Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J. Immunol. 179, 7093–7100 (2007).
Obata, K. et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110, 913–920 (2007).
Hammad, H. et al. Inflammatory dendritic cells — not basophils — are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 207, 2097–2111 (2010).
Sokol, C. L. et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nature Immunol. 10, 713–720 (2009).
Yoshimoto, T. et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nature Immunol. 10, 706–712 (2009).
Tsujimura, Y. et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity 28, 581–589 (2008).
Perrigoue, J. G. et al. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nature Immunol. 10, 697–705 (2009).
Denzel, A. et al. Basophils enhance immunological memory responses. Nature Immunol. 9, 733–742 (2008).
Paul, W. E. & Zhu, J. How are TH2-type immune responses initiated and amplified? Nature Rev. Immunol. 10, 225–235 (2010).
Kambayashi, T. et al. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J. Immunol. 182, 4686–4695 (2009).
Fox, C. C., Jewell, S. D. & Whitacre, C. C. Rat peritoneal mast cells present antigen to a PPD-specific T cell line. Cell. Immunol. 158, 253–264 (1994).
Phythian-Adams, A. T. et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J. Exp. Med. 207, 2089–2096 (2010).
Sokol, C. L., Barton, G. M., Farr, A. G. & Medzhitov, R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nature Immunol. 9, 310–318 (2008).
Sullivan, B. M. et al. Genetic analysis of basophil function in vivo. Nature Immunol. 12, 527–535 (2011).
Tang, H. et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nature Immunol. 11, 608–617 (2010).
Liang, G. et al. Naive T cells sense the cysteine protease allergen papain through protease-activated receptor 2 and propel TH2 immunity. J. Allergy Clin. Immunol. 129, 1377–1386.e13 (2012).
Khodoun, M. V., Orekhova, T., Potter, C., Morris, S. & Finkelman, F. D. Basophils initiate IL-4 production during a memory T-dependent response. J. Exp. Med. 200, 857–870 (2004).
Charles, N., Hardwick, D., Daugas, E., Illei, G. G. & Rivera, J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nature Med. 16, 701–707 (2010).
Dijkstra, D., Hennig, C., Witte, T. & Hansen, G. Basophils from humans with systemic lupus erythematosus do not express MHC-II. Nature Med. 18, 488–490 (2012).
Eckl-Dorna, J. et al. Basophils are not the key antigen-presenting cells in allergic patients. Allergy 67, 601–608 (2012).
Pulendran, B. & Artis, D. New paradigms in type 2 immunity. Science 337, 431–435 (2012).
Hepworth, M. R. et al. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc. Natl Acad. Sci. USA 109, 6644–6649 (2012).
Gauchat, J. F. et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature 365, 340–343 (1993).
Ha, T. Y., Reed, N. D. & Crowle, P. K. Immune response potential of mast cell-deficient W/Wv mice. Int. Arch. Allergy Appl. Immunol. 80, 85–94 (1986).
Wada, T. et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J. Clin. Invest. 120, 2867–2875 (2010). This study demonstrates that basophils and mast cells cooperate to provide protection against secondary infestation with ticks.
Durham, S. R., Gould, H. J. & Hamid, Q. A. Local IgE production in nasal allergy. Int. Arch. Allergy Immunol. 113, 128–130 (1997).
Bethony, J. et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367, 1521–1532 (2006).
Gause, W. C., Urban, J. F. Jr & Stadecker, M. J. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 24, 269–277 (2003).
Allen, J. E. & Maizels, R. M. Diversity and dialogue in immunity to helminths. Nature Rev. Immunol. 11, 375–388 (2011).
Anthony, R. M. et al. Protective immune mechanisms in helminth infection. Nature Rev. Immunol. 7, 975–987 (2007).
Phillips, C., Coward, W. R., Pritchard, D. I. & Hewitt, C. R. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J. Leukoc. Biol. 73, 165–171 (2003).
Machado, D. C., Horton, D., Harrop, R., Peachell, P. T. & Helm, B. A. Potential allergens stimulate the release of mediators of the allergic response from cells of mast cell lineage in the absence of sensitization with antigen-specific IgE. Eur. J. Immunol. 26, 2972–2980 (1996).
Katona, I. M., Urban, J. F. Jr & Finkelman, F. D. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J. Immunol. 140, 3206–3211 (1988).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Urban, J. F. Jr et al. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255–264 (1998).
Voehringer, D., Reese, T. A., Huang, X., Shinkai, K. & Locksley, R. M. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J. Exp. Med. 203, 1435–1446 (2006).
Liu, Q. et al. B cells have distinct roles in host protection against different nematode parasites. J. Immunol. 184, 5213–5223 (2010).
Ohnmacht, C. & Voehringer, D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J. Immunol. 184, 344–350 (2010). This study demonstrates that basophils contribute to the protective memory response against N. brasiliensis.
Crowle, P. K. & Reed, N. D. Rejection of the intestinal parasite Nippostrongylus brasiliensis by mast cell-deficient W/Wv anemic mice. Infect. Immun. 33, 54–58 (1981).
Hashimoto, K. et al. Immunity-mediated regulation of fecundity in the nematode Heligmosomoides polygyrus—the potential role of mast cells. Parasitology 137, 881–887 (2010).
Ha, T. Y., Reed, N. D. & Crowle, P. K. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect. Immun. 41, 445–447 (1983).
Knight, P. A., Wright, S. H., Lawrence, C. E., Paterson, Y. Y. & Miller, H. R. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 192, 1849–1856 (2000).
Shin, K. et al. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J. Immunol. 180, 4885–4891 (2008).
Gurish, M. F. et al. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J. Immunol. 172, 1139–1145 (2004).
Herbert, D. R. et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 (2004).
Anthony, R. M. et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature Med. 12, 955–960 (2006).
King, C. L. et al. Mice with a targeted deletion of the IgE gene have increased worm burdens and reduced granulomatous inflammation following primary infection with Schistosoma mansoni. J. Immunol. 158, 294–300 (1997).
Jankovic, D. et al. Fc epsilonRI-deficient mice infected with Schistosoma mansoni mount normal Th2-type responses while displaying enhanced liver pathology. J. Immunol. 159, 1868–1875 (1997).
Schramm, G. et al. Cutting edge: IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J. Immunol. 178, 6023–6027 (2007).
Sher, A., Correa-Oliveira, R., Hieny, S. & Hussain, R. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. IV. Analysis of the role of IgE antibodies and mast cells. J. Immunol. 131, 1460–1465 (1983).
Harris, N. & Gause, W. C. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 32, 80–88 (2011).
Lobos, E., Nutman, T. B., Hothersall, J. S. & Moncada, S. Elevated immunoglobulin E against recombinant Brugia malayi γ-glutamyl transpeptidase in patients with bancroftian filariasis: association with tropical pulmonary eosinophilia or putative immunity. Infect. Immun. 71, 747–753 (2003).
Turner, J. D. et al. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. 7, 990–996 (2005).
Rihet, P., Demeure, C. E., Bourgois, A., Prata, A. & Dessein, A. J. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur. J. Immunol. 21, 2679–2686 (1991).
Dunne, D. W. et al. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur. J. Immunol. 22, 1483–1494 (1992).
Bethony, J. et al. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J. 19, 1743–1745 (2005).
Mitre, E. & Nutman, T. B. IgE memory: persistence of antigen-specific IgE responses years after treatment of human filarial infections. J. Allergy Clin. Immunol. 117, 939–945 (2006). This human study provides evidence for the existance of long-lived IgE-producing plasma cells that developed in response to Brugia malayi infection and persisted for several years after de-worming and relocation to non-endemic areas.
Wikel, S. K. & Allen, J. R. Acquired resistance to ticks. I. Passive transfer of resistance. Immunology 30, 311–316 (1976).
Brown, S. J. & Askenase, P. W. Cutaneous basophil responses and immune resistance of guinea pigs to ticks: passive transfer with peritoneal exudate cells or serum. J. Immunol. 127, 2163–2167 (1981).
Allen, J. R. Tick resistance: basophils in skin reactions of resistant guinea pigs. Int. J. Parasitol. 3, 195–200 (1973).
Brown, S. J., Galli, S. J., Gleich, G. J. & Askenase, P. W. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J. Immunol. 129, 790–796 (1982).
Matsuda, H. et al. Necessity of IgE antibodies and mast cells for manifestation of resistance against larval Haemaphysalis longicornis ticks in mice. J. Immunol. 144, 259–262 (1990).
Wikel, S. K., Ramachandra, R. N., Bergman, D. K., Burkot, T. R. & Piesman, J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect. Immun. 65, 335–338 (1997).
Simons, F. E. Anaphylaxis. J. Allergy Clin. Immunol. 125, S161–S181 (2010).
Matasar, M. J. & Neugut, A. I. Epidemiology of anaphylaxis in the United States. Curr. Allergy Asthma Rep. 3, 30–35 (2003).
Oettgen, H. C. et al. Active anaphylaxis in IgE-deficient mice. Nature 370, 367–370 (1994).
Jacoby, W., Cammarata, P. V., Findlay, S. & Pincus, S. H. Anaphylaxis in mast cell-deficient mice. J. Invest.Dermatol. 83, 302–304 (1984).
Jonsson, F. et al. Mouse and human neutrophils induce anaphylaxis. J. Clin. Invest. 121, 1484–1496 (2011).
Arias, K. et al. Distinct immune effector pathways contribute to the full expression of peanut-induced anaphylactic reactions in mice. J. Allergy Clin. Immunol. 127, 1552–1561..e1 (2011).
Khodoun, M. et al. Peanuts can contribute to anaphylactic shock by activating complement. J. Allergy Clin. Immunol. 123, 342–351 (2009).
Koshino, T. et al. Identification of basophils by immunohistochemistry in the airways of post-mortem cases of fatal asthma. Clin. Exp. Allergy. 23, 919–925 (1993).
Liu, M. C. et al. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am. Rev. Respir. Dis. 144, 51–58 (1991).
Yu, M. et al. Mast cells can promote the development of multiple features of chronic asthma in mice. J. Clin. Invest. 116, 1633–1641 (2006).
Waern, I. et al. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J. Immunol. 183, 6369–6376 (2009).
Ito, Y. et al. Basophil recruitment and activation in inflammatory skin diseases. Allergy 66, 1107–1113 (2011).
Mukai, K. et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 23, 191–202 (2005). This study demonstrates that basophils rather than mast cells are required in a murine model of IgE-mediated chronic allergic inflammation of the skin.
Benoist, C. & Mathis, D. Mast cells in autoimmune disease. Nature 420, 875–878 (2002).
Secor, V. H., Secor, W. E., Gutekunst, C. A. & Brown, M. A. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J. Exp. Med. 191, 813–822 (2000).
Li, H. et al. Kit (W−sh) mice develop earlier and more severe experimental autoimmune encephalomyelitis due to absence of immune suppression. J. Immunol. 187, 274–282 (2011).
Bennett, J. L. et al. Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. J. Immunol. 182, 5507–5514 (2009).
Lee, D. M. et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 297, 1689–1692 (2002).
Zhou, J. S., Xing, W., Friend, D. S., Austen, K. F. & Katz, H. R. Mast cell deficiency in KitW−sh mice does not impair antibody-mediated arthritis. J. Exp. Med. 204, 2797–2802 (2007).
Schuerwegh, A. J. et al. Evidence for a functional role of IgE anticitrullinated protein antibodies in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 107, 2586–2591 (2010).
Anthony, R. M., Kobayashi, T., Wermeling, F. & Ravetch, J. V. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature 475, 110–113 (2011).
Charles, N. et al. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity 30, 533–543 (2009).
Trivedi, N. N., Raymond, W. W. & Caughey, G. H. Chimerism, point mutation, and truncation dramatically transformed mast cell delta-tryptases during primate evolution. J. Allergy Clin. Immunol. 121, 1262–1268 (2008).
Gallwitz, M., Reimer, J. M. & Hellman, L. Expansion of the mast cell chymase locus over the past 200 million years of mammalian evolution. Immunogenetics 58, 655–669 (2006).
Wong, G. W., Yasuda, S., Morokawa, N., Li, L. & Stevens, R. L. Mouse chromosome 17A3.3 contains 13 genes that encode functional tryptic-like serine proteases with distinct tissue and cell expression patterns. J. Biol. Chem. 279, 2438–2452 (2004).
Pejler, G., Ronnberg, E., Waern, I. & Wernersson, S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood 115, 4981–4990 (2010).
Ugajin, T. et al. Basophils preferentially express mouse Mast Cell Protease 11 among the mast cell tryptase family in contrast to mast cells. J. Leukoc. Biol. 86, 1417–1425 (2009).
Mancardi, D. A. et al. Cutting Edge: The murine high-affinity IgG receptor FcγRIV is sufficient for autoantibody-induced arthritis. J. Immunol. 186, 1899–1903 (2011).
Nigrovic, P. A. et al. Genetic inversion in mast cell-deficient Wsh mice interrupts Corin and manifests as hematopoietic and cardiac aberrancy. Am. J. Pathol. 173, 1693–1701 (2008).
Tsai, M., Grimbaldeston, M. A., Yu, M., Tam, S. Y. & Galli, S. J. Using mast cell knock-in mice to analyze the roles of mast cells in allergic responses in vivo. Chem. Immunol. Allergy 87, 179–197 (2005).
Grimbaldeston, M. A. et al. Mast cell-deficient W-sash c-kit mutant KitW−sh/W−sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 167, 835–848 (2005). This study analysed the cellular composition of the immune system in W-sash mice and determined the efficiency of mast cell reconstitution by intradermal, intraperitoneal and intravenous transfer of bone marrow-derived mast cells.
Lutzelschwab, C., Huang, M. R., Kullberg, M. C., Aveskogh, M. & Hellman, L. Characterization of mouse mast cell protease-8, the first member of a novel subfamily of mouse mast cell serine proteases, distinct from both the classical chymases and tryptases. Eur. J. Immunol. 28, 1022–1033 (1998).
Voehringer, D., Shinkai, K. & Locksley, R. M. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 20, 267–277 (2004). Gene expression profiling of ex vivo -isolated basophils and eosinophils from N. brasiliensis -infected mice.
Reynolds, D. S. et al. Isolation and molecular cloning of mast cell carboxypeptidase A. A novel member of the carboxypeptidase gene family. J. Biol. Chem. 264, 20094–20099 (1989).
Kitamura, Y., Go, S. & Hatanaka, K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood 52, 447–452 (1978).
Sloane, D. E. et al. Leukocyte immunoglobulin-like receptors: novel innate receptors for human basophil activation and inhibition. Blood 104, 2832–2839 (2004).
Suzukawa, M. et al. Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J. Immunol. 186, 5254–5260 (2011).
Okayama, Y. & Kawakami, T. Development, migration, and survival of mast cells. Immunol. Res. 34, 97–115 (2006).
Tsujimura, T. et al. Involvement of transcription factor encoded by the mi locus in the expression of c-kit receptor tyrosine kinase in cultured mast cells of mice. Blood 88, 1225–1233 (1996).
Tshori, S. & Nechushtan, H. Mast cell transcription factors--regulators of cell fate and phenotype. Biochim. Biophys. Acta 1822, 42–48 (2012).
McEuen, A. R., Buckley, M. G., Compton, S. J. & Walls, A. F. Development and characterization of a monoclonal antibody specific for human basophils and the identification of a unique secretory product of basophil activation. Lab. Invest. 79, 27–38 (1999).
Boehme, S. A. et al. Murine bone marrow-derived mast cells express chemoattractant receptor-homologous molecule expressed on T-helper class 2 cells (CRTh2). Int. Immunol. 21, 621–632 (2009).
Hirai, H. et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor Crth2. J. Exp. Med. 193, 255–261 (2001).
Bischoff, S. C. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nature Rev. Immunol. 7, 93–104 (2007).
Scholten, J. et al. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 17, 307–315 (2008).
Musch, W., Wege, A. K., Mannel, D. N. & Hehlgans, T. Generation and characterization of alpha-chymase-Cre transgenic mice. Genesis 46, 163–166 (2008).
Acknowledgements
The author thanks the members of the Voehringer laboratory, R. Maizels and P. Yu for discussions during the preparation of the manuscript and he apologizes to the researchers in the field whose work was not cited owing to space limitations. He further acknowledges the support by the European Research Council starting grant PAS_241506.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Voehringer, D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 13, 362–375 (2013). https://doi.org/10.1038/nri3427
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3427
This article is cited by
-
Single-cell analysis unveils activation of mast cells in colorectal cancer microenvironment
Cell & Bioscience (2023)
-
SCF and IL-33 regulate mouse mast cell phenotypic and functional plasticity supporting a pro-inflammatory microenvironment
Cell Death & Disease (2023)
-
Association of peripheral basophils with tumor M2 macrophage infiltration and outcomes of the anti-PD-1 inhibitor plus chemotherapy combination in advanced gastric cancer
Journal of Translational Medicine (2022)
-
New functions for basophils identified in kidney fibrosis
Nature Immunology (2022)
-
A cardioimmunologist’s toolkit: genetic tools to dissect immune cells in cardiac disease
Nature Reviews Cardiology (2022)