Abstract
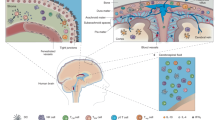
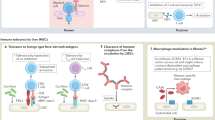
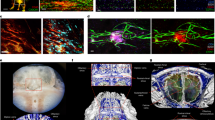
Complex barriers separate immune-privileged tissues from the circulation. Here, we propose that cell entry to immune-privileged sites through barriers composed of tight junction-interconnected endothelium is associated with destructive inflammation, whereas border structures comprised of fenestrated vasculature enveloped by tightly regulated epithelium serve as active and selective immune-skewing gates in the steady state. Based on emerging knowledge of the central nervous system and information from other immune-privileged sites, we propose that these sites are endowed either with absolute endothelial-based barriers and epithelial gates that enable selective and educative transfer of trafficking leukocytes or with selective epithelial gates only.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Rev. Immunol. 7, 678–689 (2007).
Huber, D., Balda, M. S. & Matter, K. Occludin modulates transepithelial migration of neutrophils. J. Biol. Chem. 275, 5773–5778 (2000).
Ransohoff, R. M. & Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nature Rev. Immunol. 12, 623–635 (2012).
Streilein, J. W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nature Rev. Immunol. 3, 879–889 (2003).
Naito, M. et al. The presence of intra-tubular lymphocytes in normal testis of the mouse. Okajimas Folia Anat. Jpn 85, 91–96 (2008).
Goyal, H. O. & Williams, C. S. The rete testis of the goat, a morphological study. Acta Anat. (Basel) 130, 151–157 (1987).
Nelson, J. L. The otherness of self: microchimerism in health and disease. Trends Immunol. 33, 421–427 (2012).
Takashima, S. et al. Rac mediates mouse spermatogonial stem cell homing to germline niches by regulating transmigration through the blood-testis barrier. Cell Stem Cell 9, 463–475 (2011).
Wang, C. Q. & Cheng, C. Y. A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J. Cell Biol. 178, 549–556 (2007).
Engelhardt, B. & Ransohoff, R. M. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 33, 579–589 (2012).
Alvarez, J. I. et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731 (2011).
Sonobe, Y. et al. Interleukin-25 expressed by brain capillary endothelial cells maintains blood-brain barrier function in a protein kinase Cɛ-dependent manner. J. Biol. Chem. 284, 31834–31842 (2009).
Cruz-Orengo, L. et al. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J. Exp. Med. 208, 327–339 (2011).
McCandless, E. E., Wang, Q., Woerner, B. M., Harper, J. M. & Klein, R. S. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J. Immunol. 177, 8053–8064 (2006).
Alt, C., Laschinger, M. & Engelhardt, B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 32, 2133–2144 (2002).
Galea, I. et al. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J. Exp. Med. 204, 2023–2030 (2007).
Greter, M. et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nature Med. 11, 328–334 (2005).
Ifergan, I. et al. The blood-brain barrier induces differentiation of migrating monocytes into Th17- polarizing dendritic cells. Brain 131, 785–799 (2008).
Seguin, R., Biernacki, K., Rotondo, R. L., Prat, A. & Antel, J. P. Regulation and functional effects of monocyte migration across human brain-derived endothelial cells. J. Neuropathol. Exp. Neurol. 62, 412–419 (2003).
Agrawal, S. et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 203, 1007–1019 (2006).
Shechter, R. et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity (in the press).
Provencio, J. J., Kivisakk, P., Tucky, B. H., Luciano, M. G. & Ransohoff, R. M. Comparison of ventricular and lumbar cerebrospinal fluid T cells in non-inflammatory neurological disorder (NIND) patients. J. Neuroimmunol. 163, 179–184 (2005).
Baruch, K. et al. CNS-specific immunity at the choroid plexus shifts towards destructive Th2-inflammation in brain aging. Proc. Natl Acad. Sci. USA 18 Jan 2013 (doi:10.1073/pnas.1211270110).
Kivisakk, P. et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl Acad. Sci. USA 100, 8389–8394 (2003).
Buonamici, S. et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 459, 1000–1004 (2009).
Taylor, A. W. & Streilein, J. W. Inhibition of antigen-stimulated effector T cells by human cerebrospinal fluid. Neuroimmunomodulation 3, 112–118 (1996).
Trabold, B., Rothoerl, R., Wittmann, S., Woertgen, C. & Frohlich, D. Cerebrospinal fluid and neutrophil respiratory burst after subarachnoid hemorrhage. Neuroimmunomodulation 12, 152–156 (2005).
Gordon, L. B., Nolan, S. C., Ksander, B. R., Knopf, P. M. & Harling-Berg, C. J. Normal cerebrospinal fluid suppresses the in vitro development of cytotoxic T cells: role of the brain microenvironment in CNS immune regulation. J. Neuroimmunol. 88, 77–84 (1998).
De Rotte, A. A., Verhoef, J., Andringa-Bakker, E. A. & Van Wimersma Greidanus, T. B. Characterization of the α-MSH-like immunoreactivity in blood and cerebrospinal fluid of the rat. Acta Endocrinol. 111, 440–444 (1986).
Suzuki, H. et al. Pituitary protein 7B2-like immunoreactivity in cerebrospinal fluid: comparison with other neuropeptides. J. Lab. Clin. Med. 113, 743–748 (1989).
Pentreath, V. W., Rees, K., Owolabi, O. A., Philip, K. A. & Doua, F. The somnogenic T lymphocyte suppressor prostaglandin D2 is selectively elevated in cerebrospinal fluid of advanced sleeping sickness patients. Trans. R. Soc. Trop. Med. Hyg. 84, 795–799 (1990).
Tarkowski, E., Liljeroth, A. M., Nilsson, A., Minthon, L. & Blennow, K. Decreased levels of intrathecal interleukin 1 receptor antagonist in Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 12, 314–317 (2001).
Haginoya, K. et al. Reduced levels of interleukin-1 receptor antagonist in the cerebrospinal fluid in patients with West syndrome. Epilepsy Res. 85, 314–317 (2009).
Mueller, A. M., Pedre, X., Killian, S., David, M. & Steinbrecher, A. The Decoy Receptor 3 (DcR3, TNFRSF6B) suppresses Th17 immune responses and is abundant in human cerebrospinal fluid. J. Neuroimmunol. 209, 57–64 (2009).
Pan, Y. et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 387, 611–617 (1997).
Mills, J. H. et al. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 105, 9325–9330 (2008).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Mills, J. H., Kim, D. G., Krenz, A., Chen, J. F. & Bynoe, M. S. A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J. Immunol. 188, 5713–5722 (2012).
Fujiwara, M. et al. Indoleamine 2,3-dioxygenase. Formation of L-kynurenine from L-tryptophan in cultured rabbit fineal gland. J. Biol. Chem. 253, 6081–6085 (1978).
Yamamoto, M., Drager, U. C., Ong, D. E. & McCaffery, P. Retinoid-binding proteins in the cerebellum and choroid plexus and their relationship to regionalized retinoic acid synthesis and degradation. Eur. J. Biochem. 257, 344–350 (1998).
Reboldi, A. et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nature Immunol. 10, 514–523 (2009).
Kleinewietfeld, M. et al. CCR6 expression defines regulatory effector/memory-like cells within the CD25+CD4+ T-cell subset. Blood 105, 2877–2886 (2005).
Schulz, M. & Engelhardt, B. The circumventricular organs participate in the immunopathogenesis of experimental autoimmune encephalomyelitis. Cerebrospinal Fluid Res. 266, 8 (2005).
Schmitt, C., Strazielle, N. & Ghersi-Egea, J. F. Brain leukocyte infiltration initiated by peripheral inflammation or experimental autoimmune encephalomyelitis occurs through pathways connected to the CSF-filled compartments of the forebrain and midbrain. J. Neuroinflamm. 966, 187 (2012).
Alvarez, J. I. & Teale, J. M. Differential changes in junctional complex proteins suggest the ependymal lining as the main source of leukocyte infiltration into ventricles in murine neurocysticercosis. J. Neuroimmunol. 187, 102–113 (2007).
Burton, A. R. et al. Central nervous system destruction mediated by glutamic acid decarboxylase-specific CD4+ T cells. J. Immunol. 184, 4863–4870 (2010).
Marvar, P. J. et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ. Res. 107, 263–270 (2010).
Carrithers, M. D., Visintin, I., Kang, S. J. & Janeway, C. A. Jr. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain 123, 1092–1101 (2000).
Piccio, L. et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric Gi-linked receptors. J. Immunol. 168, 1940–1949 (2002).
Derecki, N. C. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080 (2010).
Kim, J. V., Kang, S. S., Dustin, M. L. & McGavern, D. B. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457, 191–195 (2009).
Bartholomaus, I. et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98 (2009).
Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature Neurosci. 14, 1142–1149 (2011).
Kivisakk, P. et al. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann. Neurol. 65, 457–469 (2009).
Wieseler-Frank, J. et al. A novel immune-to-CNS communication pathway: cells of the meninges surrounding the spinal cord CSF space produce proinflammatory cytokines in response to an inflammatory stimulus. Brain Behav. Immun. 21, 711–718 (2007).
Kaur, C., Foulds, W. S. & Ling, E. A. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog. Retin. Eye Res. 27, 622–647 (2008).
Luna, J. D. et al. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor α, and interleukin-1β-mediated breakdown. J. Neurosci. Res. 49, 268–280 (1997).
Kerr, E. C., Copland, D. A., Dick, A. D. & Nicholson, L. B. The dynamics of leukocyte infiltration in experimental autoimmune uveoretinitis. Prog. Retin. Eye Res. 27, 527–535 (2008).
Luger, D. et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 205, 799–810 (2008).
Parnaby-Price, A. et al. Leukocyte trafficking in experimental autoimmune uveitis in vivo. J. Leukoc. Biol. 64, 434–440 (1998).
Joly, S. et al. Cooperative phagocytes: resident microglia and bone marrow immigrants remove dead photoreceptors in retinal lesions. Am. J. Pathol. 174, 2310–2323 (2009).
Sugita, S., Futagami, Y., Smith, S. B., Naggar, H. & Mochizuki, M. Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor β. Exp. Eye Res. 83, 1459–1471 (2006).
Sugita, S. Role of ocular pigment epithelial cells in immune privilege. Arch. Immunol. Ther. Exp. (Warsz) 57, 263–268 (2009).
Fang, Y., Yu, S., Ellis, J. S., Sharav, T. & Braley-Mullen, H. Comparison of sensitivity of Th1, Th2, and Th17 cells to Fas-mediated apoptosis. J. Leukoc. Biol. 87, 1019–1028 (2010).
Ishida, K., Panjwani, N., Cao, Z. & Streilein, J. W. Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul. Immunol. Inflamm. 11, 91–105 (2003).
Gregerson, D. S., Heuss, N. D., Lew, K. L., McPherson, S. W. & Ferrington, D. A. Interaction of retinal pigmented epithelial cells and CD4 T cells leads to T-cell anergy. Invest. Ophthalmol. Vis. Sci. 48, 4654–4663 (2007).
Prendergast, R. A. et al. T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest. Ophthalmol. Vis. Sci. 39, 754–762 (1998).
Sugita, S. & Streilein, J. W. Iris pigment epithelium expressing CD86 (B7-2) directly suppresses T cell activation in vitro via binding to cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 198, 161–171 (2003).
Sugita, S., Ng, T. F., Schwartzkopff, J. & Streilein, J. W. CTLA-4+CD8+ T cells that encounter B7-2+ iris pigment epithelial cells express their own B7-2 to achieve global suppression of T cell activation. J. Immunol. 172, 4184–4194 (2004).
Yoshida, M., Kezuka, T. & Streilein, J. W. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 2. Generation of TGF-β-producing regulatory T cells. Invest. Ophthalmol. Vis. Sci. 41, 3862–3870 (2000).
Sugita, S. et al. B7+ iris pigment epithelial cells convert T cells into CTLA-4+, B7-expressing CD8+ regulatory T cells. Invest. Ophthalmol. Vis. Sci. 47, 5376–5384 (2006).
Yoshida, M., Takeuchi, M. & Streilein, J. W. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 1. Inhibition of T-cell activation in vitro by direct cell-to-cell contact. Invest. Ophthalmol. Vis. Sci. 41, 811–821 (2000).
Taylor, A. W., Alard, P., Yee, D. G. & Streilein, J. W. Aqueous humor induces transforming growth factor-β (TGF-β)-producing regulatory T-cells. Curr. Eye Res. 16, 900–908 (1997).
Taylor, A. W., Yee, D. G. & Streilein, J. W. Suppression of nitric oxide generated by inflammatory macrophages by calcitonin gene-related peptide in aqueous humor. Invest. Ophthalmol. Vis. Sci. 39, 1372–1378 (1998).
Taylor, A. W., Streilein, J. W. & Cousins, S. W. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J. Immunol. 153, 1080–1086 (1994).
D'Orazio, T. J., DeMarco, B. M., Mayhew, E. S. & Niederkorn, J. Y. Effect of aqueous humor on apoptosis of inflammatory cell types. Invest. Ophthalmol. Vis. Sci. 40, 1418–1426 (1999).
Zhou, R., Horai, R., Mattapallil, M. J. & Caspi, R. R. A new look at immune privilege of the eye: dual role for the vision-related molecule retinoic acid. J. Immunol. 187, 4170–4177 (2011).
Mo, J. S. & Streilein, J. W. Immune privilege persists in eyes with extreme inflammation induced by intravitreal LPS. Eur. J. Immunol. 31, 3806–3815 (2001).
Ohta, K., Wiggert, B., Yamagami, S., Taylor, A. W. & Streilein, J. W. Analysis of immunomodulatory activities of aqueous humor from eyes of mice with experimental autoimmune uveitis. J. Immunol. 164, 1185–1192 (2000).
Ohta, K., Yamagami, S., Taylor, A. W. & Streilein, J. W. IL-6 antagonizes TGF-β and abolishes immune privilege in eyes with endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 41, 2591–2599 (2000).
Erlebacher, A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nature Rev. Immunol. 13, 23–33 (2013).
Moffett, A. & Loke, C. Immunology of placentation in eutherian mammals. Nature Rev. Immunol. 6, 584–594 (2006).
Kovats, S. et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science 248, 220–223 (1990).
Rouas-Freiss, N., Goncalves, R. M., Menier, C., Dausset, J. & Carosella, E. D. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl Acad. Sci. USA 94, 11520–11525 (1997).
Fournel, S. et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J. Immunol. 164, 6100–6104 (2000).
Carosella, E. D., Moreau, P., Aractingi, S. & Rouas-Freiss, N. HLA-G: a shield against inflammatory aggression. Trends Immunol. 22, 553–555 (2001).
Ristich, V., Liang, S., Zhang, W., Wu, J. & Horuzsko, A. Tolerization of dendritic cells by HLA-G. Eur. J. Immunol. 35, 1133–1142 (2005).
Chang, C. C. et al. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nature Immunol. 3, 237–243 (2002).
Sacks, G. P., Clover, L. M., Bainbridge, D. R., Redman, C. W. & Sargent, I. L. Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta 22, 550–559 (2001).
Liu, F. et al. Placental trophoblasts shifted Th1/Th2 balance toward Th2 and inhibited Th17 immunity at fetomaternal interface. APMIS 119, 597–604 (2011).
Schumacher, A. et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J. Immunol. 182, 5488–5497 (2009).
Siiteri, P. K. & Stites, D. P. Immunologic and endocrine interrelationships in pregnancy. Biol. Reprod. 26, 1–14 (1982).
Piccinni, M. P. et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J. Immunol. 155, 128–133 (1995).
Zorzi, W. et al. Demonstration of the expression of CD95 ligand transcript and protein in human placenta. Placenta 19, 269–277 (1998).
Jerzak, M. & Bischof, P. Apoptosis in the first trimester human placenta: the role in maintaining immune privilege at the maternal-foetal interface and in the trophoblast remodelling. Eur. J. Obstet. Gynecol. Reprod. Biol. 100, 138–142 (2002).
Hsi, B. L., Hunt, J. S. & Atkinson, J. P. Differential expression of complement regulatory proteins on subpopulations of human trophoblast cells. J. Reprod. Immunol. 19, 209–223 (1991).
Petroff, M. G. et al. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol. Reprod. 68, 1496–1504 (2003).
Suzuki, K. & Tomasi, T. B. Jr. Mechanism of immune suppression by murine neonatal fluids. J. Immunol. 125, 1806–1810 (1980).
Wilbanks, G. A. & Streilein, J. W. Fluids from immune privileged sites endow macrophages with the capacity to induce antigen-specific immune deviation via a mechanism involving transforming growth factor-β. Eur. J. Immunol. 22, 1031–1036 (1992).
Shohat, B. & Faktor, J. M. Immunosuppressive activity of human amniotic fluid of normal and abnormal pregnancies. Int. J. Fertil. 33, 273–277 (1988).
Murgita, R. A. & Tomasi, T. B. Jr. Suppression of the immune response by α-fetoprotein on the primary and secondary antibody response. J. Exp. Med. 141, 269–286 (1975).
Pressman, E. K. et al. Inflammatory cytokines and antioxidants in midtrimester amniotic fluid: correlation with pregnancy outcome. Am J. Obstet Gynecol. 204, 155.e1–155.e7 (2011).
Mital, P., Hinton, B. T. & Dufour, J. M. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol. Reprod. 84, 851–858 (2011).
Fijak, M. & Meinhardt, A. The testis in immune privilege. Immunol. Rev. 213, 66–81 (2006).
Mital, P., Kaur, G. & Dufour, J. M. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction 139, 495–504 (2010).
Suarez-Pinzon, W. et al. Testicular sertoli cells protect islet β-cells from autoimmune destruction in NOD mice by a transforming growth factor-β1-dependent mechanism. Diabetes 49, 1810–1818 (2000).
Sipione, S. et al. Identification of a novel human granzyme B inhibitor secreted by cultured sertoli cells. J. Immunol. 177, 5051–5058 (2006).
Liva, S. M. & Voskuhl, R. R. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 167, 2060–2067 (2001).
Skinner, M. K. & Moses, H. L. Transforming growth factor β gene expression and action in the seminiferous tubule: peritubular cell-Sertoli cell interactions. Mol. Endocrinol. 3, 625–634 (1989).
Gerdprasert, O. et al. Expression of monocyte chemoattractant protein-1 and macrophage colony-stimulating factor in normal and inflamed rat testis. Mol. Hum. Reprod. 8, 518–524 (2002).
Piquet-Pellorce, C., Dorval-Coiffec, I., Pham, M. D. & Jegou, B. Leukemia inhibitory factor expression and regulation within the testis. Endocrinology 141, 1136–1141 (2000).
Breucker, H. Macrophages, a normal component in seasonally involuting testes of the swan, Cygnus olor. Cell Tissue Res. 193, 463–471 (1978).
Naito, M. & Itoh, M. Patterns of infiltration of lymphocytes into the testis under normal and pathological conditions in mice. Am. J. Reprod. Immunol. 59, 55–61 (2008).
Naito, M. et al. Histopathology of the tubuli recti at the start of experimental autoimmune orchitis in mice. Med. Mol. Morphol. 42, 230–235 (2009).
Schmorl, C. in Pathologisch-anatomische Untersuchungen uber Puerperal-Eklampsie (Verlag von F.C.W. Vogel, 1893).
Reynolds, A. G. Placental metastasis from malignant melanoma; report of a case. Obstet. Gynecol. 6, 205–209 (1955).
Schroder, J. & De la Chapelle, A. Fetal lymphocytes in the maternal blood. Blood 39, 153–162 (1972).
Loubiere, L. S. et al. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab Invest. 86, 1185–1192 (2006).
Khosrotehrani, K., Johnson, K. L., Cha, D. H., Salomon, R. N. & Bianchi, D. W. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA 292, 75–80 (2004).
Srivatsa, B. Srivatsa, S., Johnson, K. L. & Bianchi, D. W. Maternal cell microchimerism in newborn tissues. J. Pediatr. 142, 31–35 (2003).
O'Donoghue, K. et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet 364, 179–182 (2004).
Evans, P. C. et al. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood 93, 2033–2037 (1999).
Nelson, J. L. et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet β cell microchimerism. Proc. Natl Acad. Sci. USA 104, 1637–1642 (2007).
Maloney, S. et al. Microchimerism of maternal origin persists into adult life. J. Clin. Invest. 104, 41–47 (1999).
Isoda, T. et al. Immunologically silent cancer clone transmission from mother to offspring. Proc. Natl Acad. Sci. USA 106, 17882–17885 (2009).
Stevens, A. M., Hermes, H. M., Rutledge, J. C., Buyon, J. P. & Nelson, J. L. Myocardial-tissue-specific phenotype of maternal microchimerism in neonatal lupus congenital heart block. Lancet 362, 1617–1623 (2003).
Mold, J. E. et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322, 1562–1565 (2008).
Nijagal, A. et al. Maternal T cells limit engraftment after in utero hematopoietic cell transplantation in mice. J. Clin. Invest. 121, 582–592 (2011).
Burlingham, W. J. A lesson in tolerance--maternal instruction to fetal cells. N. Engl. J. Med. 360, 1355–1357 (2009).
Chen, C. P. et al. Trafficking of multipotent mesenchymal stromal cells from maternal circulation through the placenta involves vascular endothelial growth factor receptor-1 and integrins. Stem Cells 26, 550–561 (2008).
Chadwick, V. S. et al. Production of peptides inducing chemotaxis and lysosomal enzyme release in human neutrophils by intestinal bacteria in vitro and in vivo. Scand. J. Gastroenterol. 23, 121–128 (1988).
Arques, J. L. et al. Salmonella induces flagellin- and MyD88-dependent migration of bacteria-capturing dendritic cells into the gut lumen. Gastroenterology 137, 579–587.e2 (2009).
Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 32, 256–264 (2011).
Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nature Immunol. 6, 507–514 (2005).
He, B. et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 (2007).
Xu, W. et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nature Immunol. 8, 294–303 (2007).
Iliev, I. D. et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58, 1481–1489 (2009).
Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nature Rev. Immunol. 12, 352–366 (2012).
Mustafa, T., Mogga, S. J., Mfinanga, S. G., Morkve, O. & Sviland, L. Immunohistochemical analysis of cytokines and apoptosis in tuberculous lymphadenitis. Immunology 117, 454–462 (2006).
Popov, A. et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J. Clin. Invest. 116, 3160–3170 (2006).
Shields, J. D., Kourtis, I. C., Tomei, A. A., Roberts, J. M. & Swartz, M. A. Induction of lymphoid-like stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752 (2010).
Acknowledgements
We thank S. Schwarzbaum for editing the manuscript. M.S. holds the Maurice and Ilse Katz Professorial Chair in Neuroimmunology. This study was funded by the European Research Council Advanced grant and the FP7-HEALTH-2011 two-stage grant given to M.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Alternatively activated macrophages
-
(M2 macrophages). Macrophages that are stimulated by interleukin-4 (IL-4) or IL-13 and that express arginase 1, mannose receptor CD206 and IL-4 receptor. Other factors may also drive the alternative activation of macrophages. M2 macrophages have an anti-inflammatory function and mediate wound healing.
- Amniotic sac
-
The sac in which the fetus develops. The sac is composed of a pair of tough but thin membranes: the inner membrane (the amnion) contains the amniotic fluid and the fetus, whereas the outer layer (the chorion) is part of the placenta.
- Aqueous humour
-
Transparent gelatinous fluid that is similar to plasma and is secreted from the non-pigmented ciliary epithelium of the eye. It circulates from behind the iris (posterior chamber), where it is formed, to the front of the iris (anterior chamber), where it drains through the trabecular meshwork into Schlemm's canal, which is a venous sinus.
- Central canal
-
A cerebrospinal fluid-filled tube that runs along the spinal cord and is continuous with the brain ventricular system.
- Choroid plexus
-
A microvilli-enriched epithelioid structure within the roof of each one of the brain ventricles that creates a surface area comparable to that of the blood–brain barrier; its most well-characterized function is the production of cerebrospinal fluid, a 'clear' plasma fluid ultrafiltrate.
- Ciliary body
-
A villous structure that is located behind the iris in the eye and produces the aqueous humour. Its stroma is coated by a double layer of ciliary epithelium; the inner layer is transparent, whereas the outer one is pigmented and forms a continuous layer with the retinal pigmented epithelium.
- Circumventricular organs
-
Structures in the brain (including the area postrema, the subfornical organ, the organum vasculosum of the lamina terminalis and the median eminence) that, owing to their neuroendocrine functions, are considered as 'windows to the brain'. They contain fenestrated endothelium, are located at strategic positions in the ventricular system and are separated from the cerebrospinal fluid by a specialized blood–cerebrospinal fluid barrier.
- Decidua
-
The specialized endometrial stromal tissue that encases the implanted conceptus. The decidua is predominantly comprised of decidual stromal cells, which differentiate from endometrial stromal cells following embryo implantation in the mouse. The decidua also contains various types of maternal leukocytes and makes direct contact with the trophoblasts on the outer surface of the conceptus to form the maternal–fetal interface.
- Ependymal cells
-
The ependyma is a thin epithelial layer that lines the ventricular system of the brain and the central canal of the spinal cord. Ependymal cells are specialized cuboidal epithelial cells that contain cilia on their apical surfaces, which circulate the cerebrospinal fluid.
- Glia limitans
-
An astrocyte structure that marks the border of the central nervous system parenchyma. It is composed of the parenchymal basement membrane and astrocyte endfeet, and covers the entire surface of the brain and spinal cord on external surfaces towards the leptomeningeal space (glia limitans superficialis) and internally towards the perivascular spaces (glia limitans perivascularis).
- Microchimerism
-
The presence within one individual of a small population of cells from another genetically distinct individual.
- Meninges
-
Vascularized tissue membranes that envelop superficial central nervous system areas and enclose the parenchyma. The meninges are composed of three layers: the outermost dura mater (beneath the skull), the arachnoid mater and the pia mater (the innermost layer, which is proximal to the parenchyma).
- Rete testis
-
Tubules located in the mediastinum testis that carry sperm from the seminiferous tubules to the efferent ducts, which are the initial section of the epididymis. This is the site at which sperm is concentrated and fluids are absorbed.
- Seminiferous tubule
-
A testicular structure in which meiosis and the subsequent creation of gametes (namely spermatozoa) takes place. There are two types of tubules: convoluted tubules are located towards the lateral end, whereas straight tubules are located towards the end that will exit the testis.
- Sertoli cells
-
Tall (columnar type) epithelial niche-forming cells, the main function of which is to nourish the developing sperm through the stages of spermatogenesis (the process of differentiation of stem cells into mature germ cells). They also consume the residual cytoplasm and engulf excess spermatozoa. The tight junctions of Sertoli cells form the blood–testis barrier, which separates the abluminal compartment of the seminiferous tubule from the blood.
- Subarachnoid space
-
The gap between the meningeal arachnoid membrane and the innermost pia mater. This cerebrospinal fluid-filled space is traversed by blood vessels.
- Tight junctions
-
A belt-like region of adhesion between adjacent epithelial or endothelial cells that regulates paracellular flux. Tight-junction proteins include the integral membrane proteins occludin and claudin, in association with cytoplasmic zonulaoccludin proteins.
- Tolerance
-
A term that denotes lymphocyte non-responsiveness to antigen but implies an active process rather than passive indifference.
- Trophoblasts
-
Specialized cells forming the outer layer of blastocytes; these cells develop to form most of the placenta, where they function in embryo implantation and the interaction with the decidualized maternal uterus.
Rights and permissions
About this article
Cite this article
Shechter, R., London, A. & Schwartz, M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol 13, 206–218 (2013). https://doi.org/10.1038/nri3391
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3391
This article is cited by
-
Blood–brain borders: a proposal to address limitations of historical blood–brain barrier terminology
Fluids and Barriers of the CNS (2024)
-
The brain cytokine orchestra in multiple sclerosis: from neuroinflammation to synaptopathology
Molecular Brain (2024)
-
Pathogenese der Uveitis
Spektrum der Augenheilkunde (2024)
-
Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis
Journal of Neuroinflammation (2023)
-
Natural killer cells in the central nervous system
Cell Communication and Signaling (2023)