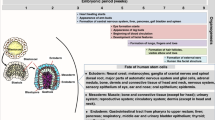
Key Points
-
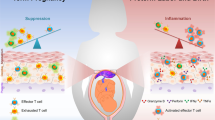
Recent work in mice has revealed several mechanisms that either minimize the activation of maternal T cells with fetal or placental specificity, or minimize the possibility that such T cells, if activated, can harm the fetus.
-
First, there is an absence of direct allorecognition of paternal MHC molecules expressed by cells of the conceptus.
-
Second, dendritic cells are trapped within the decidua, thereby minimizing the immunogenic presentation of conceptus-derived antigens in the uterine draining lymph nodes.
-
Third, regulatory T cells suppress T cell activation in response to conceptus-derived antigens.
-
Fourth, epigenetic silencing of chemokine genes occurs in decidual stromal cells, which prevents activated T cells from accumulating at the maternal–fetal interface.
Abstract
Work on the mechanisms of fetomaternal tolerance has undergone a renaissance in recent years, and the general outlines of a solution to this long-standing paradox of 'transplantation' immunology have come into view. Here, we discuss several mechanisms, recently described in mice, that either minimize the activation of maternal T cells with fetal or placental specificity, or minimize the possibility that such T cells, if activated, are able to harm the fetus. The T cell response to antigens expressed by the conceptus serves as a paradigm for the study of tissue-specific immune tolerance and is relevant to the pathogenesis of immune-mediated pregnancy complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Medawar, P. B. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 7, 320–338 (1953).
Zhang, J., Chen, Z., Smith, G. N. & Croy, B. A. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell. Mol. Immunol. 8, 1–11 (2011).
Moffett, A. & Loke, C. Immunology of placentation in eutherian mammals. Nature Rev. Immunol. 6, 584–594 (2006).
Munoz-Suano, A., Kallikourdis, M., Sarris, M. & Betz, A. G. Regulatory T cells protect from autoimmune arthritis during pregnancy. J. Autoimmun. 38, J103–J108 (2012).
Constantin, C. M. et al. Normal establishment of virus-specific memory CD8 T cell pool following primary infection during pregnancy. J. Immunol. 179, 4383–4389 (2007).
Robbins, J. R. & Bakardjiev, A. I. Pathogens and the placental fortress. Curr. Opin. Microbiol. 15, 36–43 (2012).
Munoz-Suano, A., Hamilton, A. B. & Betz, A. G. Gimme shelter: the immune system during pregnancy. Immunol. Rev. 241, 20–38 (2011).
Taglauer, E. S., Adams Waldorf, K. M. & Petroff, M. G. The hidden maternal–fetal interface: events involving the lymphoid organs in maternal–fetal tolerance. Int. J. Dev. Biol. 54, 421–430 (2010).
Nelson, J. L. The otherness of self: microchimerism in health and disease. Trends Immunol. 33, 421–427 (2012).
Game, D. S. & Lechler, R. I. Pathways of allorecognition: implications for transplantation tolerance. Transpl. Immunol. 10, 101–108 (2002).
Benichou, G., Valujskikh, A. & Heeger, P. S. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J. Immunol. 162, 352–358 (1999).
Tafuri, A., Alferink, J., Moller, P., Hammerling, G. J. & Arnold, B. T cell awareness of paternal alloantigens during pregnancy. Science 270, 630–633 (1995).
Erlebacher, A., Vencato, D., Price, K. A., Zhang, D. & Glimcher, L. H. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Invest. 117, 1399–1411 (2007). This study established the Act-mOVA mating system in mice and used this system to identify the anatomical and cellular pathways that mediate the presentation of conceptus-derived antigens to maternal T cells.
Moldenhauer, L. M. et al. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J. Immunol. 182, 8080–8093 (2009).
Ehst, B. D., Ingulli, E. & Jenkins, M. K. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am. J. Transplant. 3, 1355–1362 (2003).
Dakic, A. et al. Development of the dendritic cell system during mouse ontogeny. J. Immunol. 172, 1018–1027 (2004).
Madeja, Z. et al. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc. Natl Acad. Sci. USA 108, 4012–4017 (2011).
Redline, R. W. & Lu, C. Y. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal–fetal immunological relationship. Lab. Invest. 61, 27–36 (1989).
Mattsson, R., Mattsson, A., Holmdahl, R., Scheynius, A. & Van der Meide, P. H. In vivo treatment with interferon-γ during early pregnancy in mice induces strong expression of major histocompatibility complex class I and II molecules in uterus and decidua but not in extra-embryonic tissues. Biol. Reprod. 46, 1176–1186 (1992).
Tilburgs, T. et al. Fetal–maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J. Reprod. Immunol. 82, 148–157 (2009).
Lissauer, D., Piper, K., Goodyear, O., Kilby, M. D. & Moss, P. A. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 189, 1072–1080 (2012).
Tagliani, E. et al. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J. Exp. Med. 208, 1901–1916 (2011).
Collins, M. K., Tay, C. S. & Erlebacher, A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 119, 2062–2073 (2009). This study showed that DCs are trapped within the mouse decidua, thus precluding their involvement in the presentation of conceptus-derived antigens in the uterine draining lymph nodes.
Itano, A. A. et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003).
Allenspach, E. J., Lemos, M. P., Porrett, P. M., Turka, L. A. & Laufer, T. M. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity 29, 795–806 (2008).
Rieger, L. et al. Antigen-presenting cells in human endometrium during the menstrual cycle compared to early pregnancy. J. Soc. Gynecol. Investig. 11, 488–493 (2004).
Volchek, M. et al. Lymphatics in the human endometrium disappear during decidualization. Hum. Reprod. 25, 2455–2464 (2010).
Red-Horse, K. et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Invest. 116, 2643–2652 (2006).
Tagliani, E. & Erlebacher, A. Dendritic cell function at the maternal–fetal interface. Expert Rev. Clin. Immunol. 7, 593–602 (2011).
Rowe, J. H., Ertelt, J. M., Xin, L. & Way, S. S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490, 102–106 (2012). This study demonstrates the induction of conceptus-specific induced T Reg cells during mouse pregnancy.
Bizargity, P. & Bonney, E. A. Dendritic cells: a family portrait at mid-gestation. Immunology 126, 565–578 (2009).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Josefowicz, S. Z. et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 (2012).
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nature Immunol. 5, 266–271 (2004). Using mouse models, this study was the first to implicate a role for T Reg cells in fetomaternal tolerance.
Zenclussen, A. C. et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal–maternal interface. Eur. J. Immunol. 36, 82–94 (2006).
Mjosberg, J., Berg, G., Jenmalm, M. C. & Ernerudh, J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol. Reprod. 82, 698–705 (2010).
Dimova, T. et al. Maternal Foxp3 expressing CD4+ CD25+ and CD4+ CD25− regulatory T-cell populations are enriched in human early normal pregnancy decidua: a phenotypic study of paired decidual and peripheral blood samples. Am. J. Reprod. Immunol. 66 (Suppl. 1), 44–56 (2011).
Saito, S., Nakashima, A., Shima, T. & Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 63, 601–610 (2010).
Toldi, G. et al. The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25− FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. Am. J. Reprod. Immunol. 68, 175–180 (2012).
Samstein, R. M., Josefowicz, S. Z., Arvey, A., Treuting, P. M. & Rudensky, A. Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal–fetal conflict. Cell 150, 29–38 (2012). This study used mice deficient in induced T Reg cells to implicate a role for these cells in fetomaternal tolerance.
Moon, J. J. et al. Quantitative impact of thymic selection on Foxp3+ and Foxp3− subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc. Natl Acad. Sci. USA 108, 14602–14607 (2011).
McCloskey, M. L., Curotto de Lafaille, M. A., Carroll, M. C. & Erlebacher, A. Acquisition and presentation of follicular dendritic cell-bound antigen by lymph node-resident dendritic cells. J. Exp. Med. 208, 135–148 (2011).
Mincheva-Nilsson, L. & Baranov, V. The role of placental exosomes in reproduction. Am. J. Reprod. Immunol. 63, 520–533 (2010).
Holland, O. J. et al. Minor histocompatibility antigens are expressed in syncytiotrophoblast and trophoblast debris: implications for maternal alloreactivity to the fetus. Am. J. Pathol. 180, 256–266 (2012).
Guerin, L. R. et al. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol. Reprod. 85, 397–408 (2011).
Sharkey, D. J. et al. TGF-β mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J. Immunol. 189, 1024–1035 (2012).
Shima, T. et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 85, 121–129 (2010).
Darrasse-Jeze, G., Klatzmann, D., Charlotte, F., Salomon, B. L. & Cohen, J. L. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol. Lett. 102, 106–109 (2006).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunol. 8, 191–197 (2007).
Clark, D. A. et al. The fgl2 prothrombinase/fibroleukin gene is required for lipopolysaccharide-triggered abortions and for normal mouse reproduction. Mol. Hum. Reprod. 10, 99–108 (2004).
Falcon, B. J., Cotechini, T., Macdonald-Goodfellow, S. K., Othman, M. & Graham, C. H. Abnormal inflammation leads to maternal coagulopathies associated with placental haemostatic alterations in a rat model of foetal loss. Thromb. Haemost. 107, 438–447 (2012).
Erlebacher, A., Zhang, D., Parlow, A. F. & Glimcher, L. H. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J. Clin. Invest. 114, 39–48 (2004).
Tranguch, S. et al. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J. Clin. Invest. 117, 1824–1834 (2007).
Bizargity, P., Del Rio, R., Phillippe, M., Teuscher, C. & Bonney, E. A. Resistance to lipopolysaccharide-induced preterm delivery mediated by regulatory T cell function in mice. Biol. Reprod. 80, 874–881 (2009).
Rowe, J. H., Ertelt, J. M., Aguilera, M. N., Farrar, M. A. & Way, S. S. Foxp3+ regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe 10, 54–64 (2011).
Erlebacher, A. Immune surveillance of the maternal/fetal interface: controversies and implications. Trends Endocrinol. Metab. 21, 428–434 (2010).
Guleria, I. et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202, 231–237 (2005).
Munn, D. H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998).
Huang, L., Baban, B., Johnson, B. A. & Mellor, A. L. Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int. Rev. Immunol. 29, 133–155 (2010).
Katz, J. B., Muller, A. J. & Prendergast, G. C. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol. Rev. 222, 206–221 (2008).
Baban, B. et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 61, 67–77 (2004).
Habicht, A. et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J. Immunol. 179, 5211–5219 (2007).
Taglauer, E. S., Yankee, T. M. & Petroff, M. G. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J. Reprod. Immunol. 80, 12–21 (2009).
Svensson, L., Arvola, M., Sallstrom, M. A., Holmdahl, R. & Mattsson, R. The Th2 cytokines IL-4 and IL-10 are not crucial for the completion of allogeneic pregnancy in mice. J. Reprod. Immunol. 51, 3–7 (2001).
Robertson, S. A., Care, A. S. & Skinner, R. J. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol. Reprod. 76, 738–748 (2007).
Murphy, S. P., Fast, L. D., Hanna, N. N. & Sharma, S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J. Immunol. 175, 4084–4090 (2005).
Reinhardt, R. L., Bullard, D. C., Weaver, C. T. & Jenkins, M. K. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J. Exp. Med. 197, 751–762 (2003).
Masopust, D. et al. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 172, 4875–4882 (2004).
Kallikourdis, M., Andersen, K. G., Welch, K. A. & Betz, A. G. Alloantigen-enhanced accumulation of CCR5+ 'effector' regulatory T cells in the gravid uterus. Proc. Natl Acad. Sci. USA 104, 594–599 (2007).
Blois, S. M. et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nature Med. 13, 1450–1457 (2007).
Nancy, P. et al. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal–fetal interface. Science 336, 1317–1321 (2012). This study demonstrates the existence of an epigenetic programme of chemokine gene silencing that prevents the accumulation of activated T cells in the mouse decidua.
Margueron, R. & Reinberg, D. The Polycomb complex PRC2 and its mark in life. Nature 469, 343–349 (2011).
Vassiliadou, N. & Bulmer, J. N. Quantitative analysis of T lymphocyte subsets in pregnant and nonpregnant human endometrium. Biol. Reprod. 55, 1017–1022 (1996).
Kim, C. J. et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod. Pathol. 23, 1000–1011 (2010).
Edmondson, N. et al. The prevalence of chronic deciduitis in cases of preterm labor without clinical chorioamnionitis. Pediatr. Dev. Pathol. 12, 16–21 (2009).
Redline, R. W. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum. Pathol. 38, 1439–1446 (2007).
Langley-Evans, S. C. & McMullen, S. Developmental origins of adult disease. Med. Princ. Pract. 19, 87–98 (2010).
Acknowledgements
Work in the author's laboratory has been supported by grants from the US National Institutes of Health, the American Cancer Society and the Leona M. and Harry B. Helmsley Charitable Trust. The author would like to thank the members of his laboratory for many stimulating discussions.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Pre-eclampsia
-
A disease specific to pregnancy that is triggered by placental dysfunction. It is characterized by diverse systemic symptoms, including hypertension and proteinuria. The incidence varies from 3% to 10% of pregnancies, and this disease is the leading cause of maternal mortality and fetal morbidity and mortality throughout the world.
- Immune privilege
-
Immune-privileged sites are areas in the body with a decreased immune response to foreign antigens, including tissue grafts. Classic sites of immune privilege include the brain, eyes and testes.
- Haemochorial mode of placentation
-
A form of placentation in which trophoblast cells erode the maternal vasculature, which results in the direct contact of maternal blood with trophoblasts.
- Decidua
-
The specialized endometrial stromal tissue that encases the implanted conceptus. The decidua is predominantly comprised of decidual stromal cells, which differentiate from endometrial stromal cells following embryo implantation in the mouse. The decidua also contains various types of maternal leukocytes, and it makes direct contact with the trophoblasts on the outer surface of the conceptus to form the maternal–fetal interface.
- Trophoblasts
-
The earliest extra-embryonic cells to differentiate from the cells of the mammalian embryo. They constitute the predominant cellular component of the placenta, surround the conceptus throughout gestation and make direct contact with maternal tissues.
- Minor histocompatibility antigens
-
Polymorphic peptides derived from normal cellular proteins that can be recognized by T cells when presented on MHC molecules. Immune responses against these polymorphic antigens can result in graft-versus-host reactions, graft rejection or beneficial antitumour responses.
- Tetramer
-
A reagent comprised of a fluorophore-conjugated core surrounded by four peptide—MHC complexes or ligand–CD1d complexes. In reality, these are much more than tetramers, because each of the four complexes involved comprises multiple components, and higher-order associations can also occur. Thus, 'oligomer' might be a more accurate term.
- Exosome
-
A small lipid-bilayer vesicle that is released from activated cells following the fusion of a multivesicular body with the plasma membrane.
- Indoleamine 2,3-dioxygenase
-
(IDO). An intracellular haem-containing enzyme that catalyses the oxidative catabolism of tryptophan. The activity of IDO reduces the availability of tryptophan, which can lead to T cell apoptosis and anergy.
- TC1 cells
-
CD8+ cytotoxic T cells that produce T helper 1-type cytokines, particularly interferon-γ.
Rights and permissions
About this article
Cite this article
Erlebacher, A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol 13, 23–33 (2013). https://doi.org/10.1038/nri3361
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3361
This article is cited by
-
Prominent epigenetic and transcriptomic changes in CD4+ and CD8+ T cells during and after pregnancy in women with multiple sclerosis and controls
Journal of Neuroinflammation (2023)
-
Establishment of fetomaternal tolerance through glycan-mediated B cell suppression
Nature (2022)
-
Syncytin-1 nonfusogenic activities modulate inflammation and contribute to preeclampsia pathogenesis
Cellular and Molecular Life Sciences (2022)
-
Transcriptomic analysis of equine chorioallantois reveals immune networks and molecular mechanisms involved in nocardioform placentitis
Veterinary Research (2021)
-
Direct and Indirect endocrine-mediated suppression of human endometrial CD8+T cell cytotoxicity
Scientific Reports (2021)