Key Points
-
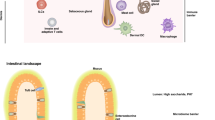
Mammalian intestine and skin interface with the external environment and are thus in contact with both pathogenic and commensal microorganisms. Antimicrobial proteins produced by epithelial cells are essential for defending against microbial challenges at these tissue sites.
-
Mammalian antimicrobial proteins are members of a diverse array of protein families and kill microorganisms through various mechanisms, including enzymatic attack and membrane disruption.
-
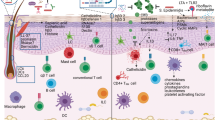
Several antimicrobial proteins also function as potent immune regulators that modulate downstream immune responses to microorganisms.
-
The expression, secretion and activity of antimicrobial proteins are tightly controlled. Regulation is multifaceted, encompassing both transcriptional and post-translational mechanisms.
-
Antimicrobial proteins function in vivo to limit pathogen colonization, to determine microbiota composition and to restrict microbiota access to host tissues.
-
Dysregulation of antimicrobial protein function is associated with diseases of the intestine and skin. These include inflammatory bowel disease and skin disorders such as atopic dermatitis, rosacea and psoriasis.
Abstract
Surface tissues of the body such as the skin and intestinal tract are in direct contact with the external environment and are thus continuously exposed to large numbers of microorganisms. To cope with the substantial microbial exposure, epithelial surfaces produce a diverse arsenal of antimicrobial proteins that directly kill or inhibit the growth of microorganisms. In this Review, we highlight new advances in our understanding of how epithelial antimicrobial proteins protect against pathogens and contribute to microbiota–host homeostasis at the skin and gut mucosae. Further, we discuss recent insights into the regulatory mechanisms that control antimicrobial protein expression. Finally, we consider how impaired antimicrobial protein expression and function can contribute to disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Neish, A. S. The gut microflora and intestinal epithelial cells: a continuing dialogue. Microbes Infect. 4, 309–317 (2002).
Grice, E. A. & Segre, J. A. The skin microbiome. Nature Rev. Microbiol. 9, 244–253 (2011).
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002).
Mukherjee, S., Vaishnava, S. & Hooper, L. V. Multi-layered regulation of intestinal antimicrobial defense. Cell. Mol. Life Sci. 65, 3019–3027 (2008).
Lai, Y. & Gallo, R. L. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 30, 131–141 (2009).
Gläser, R. et al. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nature Immunol. 6, 57–64 (2005).
Schittek, B. et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nature Immunol. 2, 1133–1137 (2001).
Ouellette, A. J. Paneth cell α-defensins in enteric innate immunity. Cell. Mol. Life Sci. 68, 2215–2229 (2011).
Christa, L. et al. HIP/PAP is an adhesive protein expressed in hepatocarcinoma, normal Paneth, and pancreatic cells. Am. J. Physiol. 271, G993–G1002 (1996).
Ogawa, H. et al. Increased expression of HIP/PAP and regenerating gene III in human inflammatory bowel disease and a murine bacterial reconstitution model. Inflamm. Bowel Dis. 9, 162–170 (2003).
Cash, H. L., Whitham, C. V., Behrendt, C. L. & Hooper, L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006).
Lehotzky, R. E. et al. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proc. Natl Acad. Sci. USA 107, 7722–7727 (2010).
Gallo, R. L. et al. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl Acad. Sci. USA 91, 11035–11039 (1994).
Harder, J., Bartels, J., Christophers, E. & Schröder, J. M. A peptide antibiotic from human skin. Nature 387, 861 (1997).
Larrick, J. W. et al. Structural, functional analysis and localization of the human CAP18 gene. FEBS Lett. 398, 74–80 (1996).
Gudmundsson, G. H. et al. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238, 325–332 (1996).
Gallo, R. L. et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 272, 13088–13093 (1997).
Di Nardo, A., Vitiello, A. & Gallo, R. L. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 170, 2274–2278 (2003).
Murakami, M. et al. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J. Invest. Dermatol. 119, 1090–1095 (2002).
Frohm, M. et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272, 15258–15263 (1997).
Dorschner, R. A. et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Invest. Dermatol. 117, 91–97 (2001).
Vaishnava, S., Behrendt, C. L., Ismail, A. S., Eckmann, L. & Hooper, L. V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl Acad. Sci. USA 105, 20858–20863 (2008).
Vaishnava, S. et al. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011). This paper shows that the antibacterial protein REG3γ limits direct contact between the intestinal microbiota and host tissues, and thus helps to preserve the symbiotic nature of the host–microbiota relationship.
O'Neil, D. A. et al. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163, 6718–6724 (1999).
Hase, K., Eckmann, L., Leopard, J. D., Varki, N. & Kagnoff, M. F. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect. Immun. 70, 953–963 (2002).
Hooper, L. V., Stappenbeck, T. S., Hong, C. V. & Gordon, J. I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nature Immunol. 4, 269–273 (2003).
Johansson, M. E. V. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Meyer-Hoffert, U. et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 57, 764–771 (2008).
Emelianov, V. U. et al. Immunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversa. Br. J. Dermatol. 166, 1023–1034 (2012).
Frohm Nilsson, M. et al. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 67, 2561–2566 (1999).
Sørensen, O. E. et al. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J. Immunol. 170, 5583–5589 (2003).
Wang, Z. et al. Skin mast cells protect mice against vaccinia virus by triggering mast cell receptor S1PR2 and releasing antimicrobial peptides. J. Immunol. 188, 345–357 (2012).
Harwig, S. S. et al. Bactericidal properties of murine intestinal phospholipase A2. J. Clin. Invest. 95, 603–610 (1995).
Koprivnjak, T., Peschel, A., Gelb, M. H., Liang, N. S. & Weiss, J. P. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 277, 47636–47644 (2002).
Kagan, B. L., Selsted, M. E., Ganz, T. & Lehrer, R. I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl Acad. Sci. USA 87, 210–214 (1990).
Bals, R. & Wilson, J. M. Cathelicidins — a family of multifunctional antimicrobial peptides. Cell. Mol. Life Sci. 60, 711–720 (2003).
Gennaro, R., Zanetti, M., Benincasa, M., Podda, E. & Miani, M. Pro-rich antimicrobial peptides from animals: structure, biological functions and mechanism of action. Curr. Pharm. Des. 8, 763–778 (2002).
Harder, J. & Schroder, J.-M. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 277, 46779–46784 (2002).
Corbin, B. D. et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965 (2008).
Kehl-Fie, T. E. et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10, 158–164 (2011).
De Yang. et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192, 1069–1074 (2000).
Kurosaka, K., Chen, Q., Yarovinsky, F., Oppenheim, J. J. & Yang, D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 174, 6257–6265 (2005).
Yang, D., Biragyn, A., Kwak, L. W. & Oppenheim, J. J. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23, 291–296 (2002).
Niyonsaba, F. et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106, 20–26 (2002).
Yang, D., Chertov, O. & Oppenheim, J. J. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J. Leukoc. Biol. 69, 691–697 (2001).
Yang, D. et al. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286, 525–528 (1999).
Tokumaru, S. et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 175, 4662–4668 (2005).
Yamasaki, K. et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nature Med. 13, 975–980 (2007).
Di Nardo, A. et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 178, 1829–1834 (2007).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007). This paper describes the discovery of a novel mechanism by which antimicrobial peptides can break tolerance to self-DNA.
Falk, P. G., Hooper, L. V., Midtvedt, T. & Gordon, J. I. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 62, 1157–1170 (1998).
van Es, J. H. et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nature Cell Biol. 7, 381–386 (2005).
Putsep, K. et al. Germ-free and colonized mice generate the same products from enteric prodefensins. J. Biol. Chem. 275, 40478–40482 (2000).
Hooper, L. V. et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001).
Abtin, A. et al. Flagellin is the principal inducer of the antimicrobial peptide S100A7c (psoriasin) in human epidermal keratinocytes exposed to Escherichia coli. FASEB J. 22, 2168–2176 (2008).
Brandl, K., Plitas, G., Schnabl, B., DeMatteo, R. P. & Pamer, E. G. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. J. Exp. Med. 204, 1891–1900 (2007).
Rakoff-Nahoum, S. & Medzhitov, R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 317, 124–127 (2007).
Kinnebrew, M. A. et al. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J. Infect. Dis. 201, 534–543 (2010).
Schauber, J. et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut 52, 735–741 (2003).
Sanos, S. L., Vonarbourg, C., Mortha, A. & Diefenbach, A. Control of epithelial cell function by interleukin-22-producing RORγt+ innate lymphoid cells. Immunology 132, 453–465 (2011).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Sanos, S. L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nature Immunol. 10, 83–91 (2008).
Ogura, Y. et al. Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut 52, 1591–1597 (2003).
Girardin, S. E. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003).
Petnicki-Ocwieja, T. et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl Acad. Sci. USA 106, 15813–15818 (2009). This study shows that the PRR NOD2 controls microbiota load and composition in the small intestine.
Kobayashi, K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005).
Mackie, R. I., Sghir, A. & Gaskins, H. R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 69, 1035S–1045S (1999).
Ménard, S. et al. Developmental switch of intestinal antimicrobial peptide expression. J. Exp. Med. 205, 183–193 (2008).
Iimura, M. et al. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J. Immunol. 174, 4901–4907 (2005).
Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl Acad. Sci. USA 84, 5449–5453 (1987). This landmark paper describes the early identification of an antimicrobial peptide in frog skin.
Lambert, J. et al. Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides. Proc. Natl Acad. Sci. USA 86, 262–266 (1989).
Aberg, K. M. et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J. Invest. Dermatol. 128, 917–925 (2008).
Sørensen, O. E. et al. Injury-induced innate immune response in human skin mediated by transactivation of the epidermal growth factor receptor. J. Clin. Invest. 116, 1878–1885 (2006).
Wang, T.-T. et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 173, 2909–2912 (2004).
Gombart, A. F., Borregaard, N. & Koeffler, H. P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3 . FASEB J. 19, 1067–1077 (2005).
Liu, P. T. et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311, 1770–1773 (2006). This paper linked control of antimicrobial peptide expression by vitamin D to susceptibility to tuberculosis.
Schauber, J. et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 117, 803–811 (2007).
Hata, T. R. et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J. Allergy Clin. Immunol. 122, 829–831 (2008).
Hong, S. P. et al. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J. Invest. Dermatol. 128, 2880–2887 (2008).
Lichtenstein, A., Ganz, T., Selsted, M. E. & Lehrer, R. I. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood 68, 1407–1410 (1986).
Wilson, C. L. et al. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286, 113–117 (1999).
Ghosh, D. et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nature Immunol. 3, 583–590 (2002).
Mukherjee, S. et al. Regulation of C-type lectin antimicrobial activity by a flexible N-terminal prosegment. J. Biol. Chem. 284, 4881–4888 (2009).
Sørensen, O., Arnljots, K., Cowland, J. B., Bainton, D. F. & Borregaard, N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90, 2796–2803 (1997).
Yamasaki, K. et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 20, 2068–2080 (2006).
Schutte, B. C. & McCray, P. B. β-defensins in lung host defense. Annu. Rev. Physiol. 64, 709–748 (2002).
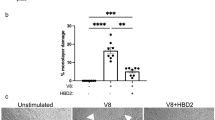
Schroeder, B. O. et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469, 419–423 (2011). This study provides new insights into the regulation of antimicrobial protein function by showing that the reducing environment of the intestine is required for the full expression of antimicrobial activity in a human β-defensin.
Ayabe, T. et al. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nature Immunol. 1, 113–118 (2000).
Salzman, N. H., Ghosh, D., Huttner, K. M., Paterson, Y. & Bevins, C. L. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422, 522–526 (2003). This paper provides insights into the in vivo function of α-defensins by showing that DEFA5 limits pathogen colonization of the intestine.
Howell, M. D. et al. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J. Immunol. 172, 1763–1767 (2004).
Kulkarni, M. M. et al. Mammalian antimicrobial peptide influences control of cutaneous Leishmania infection. Cell. Microbiol. 13, 913–923 (2011).
Nizet, V. et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457 (2001). This paper was the first direct demonstration that an antimicrobial peptide is essential for mammalian immune defence.
Chromek, M. et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nature Med. 12, 636–641 (2006).
Huang, L. C., Reins, R. Y., Gallo, R. L. & McDermott, A. M. Cathelicidin-deficient (Cnlp−/−) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 48, 4498–4508 (2007).
Brandl, K. et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455, 804–807 (2008).
Salzman, N. H. et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nature Immunol. 11, 76–83 (2010). This study showed for the first time that antimicrobial proteins can regulate the composition of intestinal microbial communities.
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Cogen, A. L. et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Invest. Dermatol. 130, 192–200 (2010).
Iwase, T. et al. Staphylococcus epidermidis Esp. inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465, 346–349 (2010).
Otto, M., Süssmuth, R., Vuong, C., Jung, G. & Götz, F. Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett. 450, 257–262 (1999).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Swidsinski, A., Weber, J., Loening-Baucke, V., Hale, L. P. & Lochs, H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 43, 3380–3389 (2005).
Koslowski, M. J. et al. Genetic variants of Wnt transcription factor TCF-4 (TCF7L2) putative promoter region are associated with small intestinal Crohn's disease. PLoS ONE 4, e4496 (2009).
Koslowski, M. J. et al. Association of a functional variant in the Wnt co-receptor LRP6 with early onset ileal Crohn's disease. PLoS Genet. 8, e1002523 (2012).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411, 603–606 (2001).
Wehkamp, J. et al. Reduced Paneth cell α-defensins in ileal Crohn's disease. Proc. Natl Acad. Sci. USA 102, 18129–18134 (2005).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008). This paper demonstrates that autophagy pathways regulate the exocytosis of Paneth cell secretory granules and provides insights into how perturbations in the autophagy pathway might lead to IBD.
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Garabedian, E. M., Roberts, L. J., McNevin, M. S. & Gordon, J. I. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J. Biol. Chem. 272, 23729–23740 (1997).
Zanger, P. et al. Constitutive expression of the antimicrobial peptide RNase 7 is associated with Staphylococcus aureus infection of the skin. J. Infect. Dis. 200, 1907–1915 (2009).
Zanger, P. et al. Severity of Staphylococcus aureus infection of the skin is associated with inducibility of human β-defensin 3 but not human β-defensin 2. Infect. Immun. 78, 3112–3117 (2010).
Ong, P. Y. et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347, 1151–1160 (2002).
de Jongh, G. J. et al. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J. Invest. Dermatol. 125, 1163–1173 (2005).
Kisich, K. O., Carspecken, C. W., Fiéve, S., Boguniewicz, M. & Leung, D. Y. M. Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human β-defensin-3. J. Allergy Clin. Immunol. 122, 62–68 (2008).
Hata, T. R. et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br. J. Dermatol. 163, 659–661 (2010).
Howell, M. D. et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 24, 341–348 (2006).
Yamasaki, K. et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J. Invest. Dermatol. 131, 688–697 (2011).
Jansen, T., Krug, S., Kind, P., Plewig, G. & Messer, G. BsmI polymorphism of the vitamin D receptor gene in patients with the fulminant course of rosacea conglobata (rosacea fulminans). J. Dermatol. 31, 244–246 (2004).
Peschel, A. et al. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274, 8405–8410 (1999).
Gunn, J. S., Ryan, S. S., Van Velkinburgh, J. C., Ernst, R. K. & Miller, S. I. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar typhimurium. Infect. Immun. 68, 6139–6146 (2000).
Robey, M., O'Connell, W. & Cianciotto, N. P. Identification of Legionella pneumophila rcp, a pagP-like gene that confers resistance to cationic antimicrobial peptides and promotes intracellular infection. Infect. Immun. 69, 4276–4286 (2001).
Sieprawska-Lupa, M. et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob. Agents Chemother. 48, 4673–4679 (2004).
Nyberg, P., Rasmussen, M. & Björck, L. α2-macroglobulin-proteinase complexes protect Streptococcus pyogenes from killing by the antimicrobial peptide LL-37. J. Biol. Chem. 279, 52820–52823 (2004).
Shafer, W. M., Qu, X., Waring, A. J. & Lehrer, R. I. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl Acad. Sci. USA 95, 1829–1833 (1998).
Islam, D. et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nature Med. 7, 180–185 (2001).
Koprivnjak, T. & Peschel, A. Bacterial resistance mechanisms against host defense peptides. Cell. Mol. Life Sci. 68, 2243–2254 (2011).
Nizet, V. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J. Allergy Clin. Immunol. 120, 13–22 (2007).
Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Ley, R. E. et al. Evolution of mammals and their gut microbes. Science 320, 1647–1651 (2008).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009).
Sumikawa, Y. et al. Induction of β-defensin 3 in keratinocytes stimulated by bacterial lipopeptides through toll-like receptor 2. Microbes Infect. 8, 1513–1521 (2006).
Sass, V. et al. Human β-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect. Immun. 78, 2793–2800 (2010).
Stowell, S. R. et al. Innate immune lectins kill bacteria expressing blood group antigen. Nature Med. 16, 295–302 (2010).
Flo, T. H. et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004).
Raffatellu, M. et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5, 476–486 (2009).
Müller, C. A., Autenrieth, I. B. & Peschel, A. Innate defenses of the intestinal epithelial barrier. Cell. Mol. Life Sci. 62, 1297–1307 (2005).
Royet, J., Gupta, D. & Dziarski, R. Peptidoglycan recognition proteins: modulators of the microbiome and inflammation. Nature Rev. Immunol. 11, 837–851 (2011).
Qu, X. D. & Lehrer, R. I. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect. Immun. 66, 2791–2797 (1998).
Acknowledgements
R.L.G. and L.V.H. thank their students and colleagues for the many discussions that contributed to the ideas in this manuscript. Work in R.L.G.'s laboratory is supported by US National Institutes of Health grants AR052728, AI052453, AI083358, contract HHSN272201000020C and a Merit Award from the Veterans Administration. Work in L.V.H.'s laboratory is supported by the Howard Hughes Medical Institute, the US National Institutes of Health (DK070855), the Burroughs Wellcome Foundation and the Crohn's and Colitis Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Commensal microorganisms
-
The microorganisms that are present in normal, healthy individuals. These microorganisms live in the gastrointestinal tract and at other body sites, and generally engage in mutually beneficial relationships with their hosts.
- C-type lectins
-
A large family of receptors that have carbohydrate recognition domains. The designation 'C-type' is based on the structure of the carbohydrate recognition domain. Several epithelial antimicrobial proteins, including regenerating islet-derived protein 3γ (REG3γ) and hepatointestinal pancreatic/pancreatitis-associated protein (HIP/PAP), are members of the C-type lectin family.
- Ribonucleases
-
(RNases). Enzymes that catalyse the breakdown of RNA. Several antimicrobial proteins (for example, RNase7 and angiogenin 4) have RNase activity, although the significance of this for the antibacterial activity of these proteins is not known.
- Cryptdins
-
Mouse α-defensins are frequently designated as 'cryptdins', which stands for 'crypt α-defensins'.
- Peptidoglycan
-
A polymer of sugars, crosslinked by short peptides, that is a crucial component of the bacterial cell wall.
- Enterocytes
-
Absorptive columnar epithelial cells that are the major epithelial lineage of the intestine.
- Paneth cells
-
A specialized epithelial cell lineage that produces most of the antimicrobial proteins in the small intestine.
- Crypts of Lieberkühn
-
Invaginations of the small intestinal surface that contain both Paneth cells and intestinal stem cells.
- Pattern recognition receptors
-
(PRRs). Host receptors (such as Toll-like receptors (TLRs) or NOD-like receptors (NLRs)) that can sense pathogen-associated molecular patterns and initiate signalling cascades that lead to an innate immune response. These can be membrane bound (for example, TLRs) or soluble cytoplasmic receptors (for example, RIG-I, MDA5 and NLRs).
- Germ-free animals
-
Animals that are reared in isolators, without exposure to microorganisms.
- WNT pathway
-
A signalling pathway that controls several physiological processes, including embryogenesis and cancer development. It also controls normal biological functions in adult animals and is essential for the expression of α-defensins in the small intestine.
- Conventionally raised mice
-
Mice that have been raised with normal exposure to microorganisms.
- Innate lymphoid cells
-
(ILCs). A diverse family of immune cells that produce cytokines and function to coordinate immunity and inflammation in body surface tissues such as the intestine and the lung. Although their developmental origins are still unclear, they phenotypically resemble natural killer cells.
- Climax community
-
A mature, stable community of organisms that develops through a process of ecological succession and remains in a steady state for an extended period of time.
- Anaerobes
-
Microorganisms that grow in the absence of oxygen.
- 16S ribosomal RNA gene sequencing
-
Determination of the sequences of the variable regions of bacterial ribosomal RNA genes, which are conserved within a species but differ between species. It is frequently used as a culture-independent technique for evaluating the composition of bacterial communities.
- Segmented filamentous bacteria
-
(SFB). A group of Gram-positive bacteria that are members of the intestinal microbiota of mice. They are characterized by their ability to adhere to the intestinal surface and are frequently immunostimulatory.
- Quorum sensing
-
A system used by bacteria to coordinate gene expression as a function of population density.
Rights and permissions
About this article
Cite this article
Gallo, R., Hooper, L. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12, 503–516 (2012). https://doi.org/10.1038/nri3228
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3228
This article is cited by
-
Mechanisms of medicinal, pharmaceutical, and immunomodulatory action of probiotics bacteria and their secondary metabolites against disease management: an overview
Folia Microbiologica (2024)
-
Cross-alteration of murine skin and tick microbiome concomitant with pathogen transmission after Ixodes ricinus bite
Microbiome (2023)
-
Antimicrobial protein REG3A regulates glucose homeostasis and insulin resistance in obese diabetic mice
Communications Biology (2023)
-
Mechanisms and regulation of defensins in host defense
Signal Transduction and Targeted Therapy (2023)
-
Modulatory role of vitamins A, B3, C, D, and E on skin health, immunity, microbiome, and diseases
Pharmacological Reports (2023)