Key Points
-
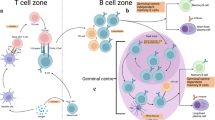
High-affinity B cell memory emerges across three separable phases of development. Each phase involves antigen recognition by specific B cells and contact with cognate T follicular helper (TFH) cells.
-
Initial commitment to the memory B cell pathway occurs before germinal centre (GC) formation, following first contact with antigen-specific TFH cells (pre-GC phase). Molecular interactions at the cellular interface involve cell-associated contacts and signals from secreted molecules such as cytokines.
-
The GC reaction supports reiterative cycles of B cell receptor (BCR) diversification, clonal expansion and class-switch recombination (GC phase) that promote the positive selection of high-affinity GC B cell variants into the memory B cell compartment.
-
Following antigen recall, memory B cells require regulation by antigen-specific TFH cells to proliferate and differentiate into memory-response plasma cells (memory phase). Affinity maturation of the antibody response continues at this stage using mechanisms that are poorly understood.
-
Antigen-specific TFH cells regulate each phase of development and consolidate memory B cell fate in high-affinity pre-memory and memory B cells.
-
Beyond antigen recognition, antibody class determines immune function and antibody affinity controls the sensitivity of memory B cells.
Abstract
The development of high-affinity B cell memory is regulated through three separable phases, each involving antigen recognition by specific B cells and cognate T helper cells. Initially, antigen-primed B cells require cognate T cell help to gain entry into the germinal centre pathway to memory. Once in the germinal centre, B cells with variant B cell receptors must access antigens and present them to germinal centre T helper cells to enter long-lived memory B cell compartments. Following antigen recall, memory B cells require T cell help to proliferate and differentiate into plasma cells. A recent surge of information — resulting from dynamic B cell imaging in vivo and the elucidation of T follicular helper cell programmes — has reshaped the conceptual landscape surrounding the generation of memory B cells. In this Review, we integrate this new information about each phase of antigen-specific B cell development to describe the newly unravelled molecular dynamics of memory B cell programming.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pape, K. A., Catron, D. M., Itano, A. A. & Jenkins, M. K. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 26, 491–502 (2007).
Roozendaal, R. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009).
Qi, H., Egen, J. G., Huang, A. Y. & Germain, R. N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312, 1672–1676 (2006).
Batista, F. D. & Harwood, N. E. The who, how and where of antigen presentation to B cells. Nature Rev. Immunol. 9, 15–27 (2009).
Carrasco, Y. R. & Batista, F. D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27, 160–171 (2007).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Phan, T. G., Green, J. A., Gray, E. E., Xu, Y. & Cyster, J. G. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nature Immunol. 10, 786–793 (2009). This study provides evidence that the initial non-cognate relay of antigens to follicular DC networks is required to support affinity maturation at later stages of the immune response.
Iannacone, M. et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 465, 1079–1083 (2010).
Fleire, S. J. et al. B cell ligand discrimination through a spreading and contraction response. Science 312, 738–741 (2006).
Randall, K. L., Lambe, T., Goodnow, C. C. & Cornall, R. J. The essential role of DOCK8 in humoral immunity. Dis. Markers 29, 141–150 (2010).
Winslow, M. M., Gallo, E. M., Neilson, J. R. & Crabtree, G. R. The calcineurin phosphatase complex modulates immunogenic B cell responses. Immunity 24, 141–152 (2006).
Khiem, D., Cyster, J. G., Schwarz, J. J. & Black, B. L. A p38 MAPK–MEF2C pathway regulates B-cell proliferation. Proc. Natl Acad. Sci. USA 105, 17067–17072 (2008).
Wilker, P. R. et al. Transcription factor Mef2c is required for B cell proliferation and survival after antigen receptor stimulation. Nature Immunol. 9, 603–612 (2008).
Matsumoto, M. et al. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity 34, 703–714 (2011).
Capasso, M. et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nature Immunol. 11, 265–272 (2010).
Damdinsuren, B. et al. Single round of antigen receptor signaling programs naive B cells to receive T cell help. Immunity 32, 355–366 (2010).
Cantor, J. et al. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nature Immunol. 10, 412–419 (2009).
Yasuda, T. et al. ERKs induce expression of the transcriptional repressor Blimp-1 and subsequent plasma cell differentiation. Sci. Signal. 4, ra25 (2011).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Fazilleau, N., Mark, L., McHeyzer-Williams, L. J. & McHeyzer-Williams, M. G. Follicular helper T cells: lineage and location. Immunity 30, 324–335 (2009).
Fazilleau, N., McHeyzer-Williams, L. J., Rosen, H. & McHeyzer-Williams, M. G. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nature Immunol. 10, 375–384 (2009).
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Nurieva, R. I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Linterman, M. A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Betz, B. C. et al. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J. Exp. Med. 207, 933–942 (2010).
Ise, W. et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nature Immunol. 12, 536–543 (2011).
Choi, Y. S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011).
Hou, B. et al. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity 34, 375–384 (2011).
Kitano, M. et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity 34, 961–972 (2011).
Qi, H., Cannons, J. L., Klauschen, F., Schwartzberg, P. L. & Germain, R. N. SAP-controlled T–B cell interactions underlie germinal centre formation. Nature 455, 764–769 (2008). An outstanding dynamic imaging study of pre-GC cognate contact between B cells and T cells that identified the functional impact of contact duration on antigen-specific B cell fate determination.
Kerfoot, S. M. et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 34, 947–960 (2011).
Stavnezer, J., Guikema, J. E. & Schrader, C. E. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 26, 261–292 (2008).
Tran, T. H. et al. B cell-specific and stimulation-responsive enhancers derepress Aicda by overcoming the effects of silencers. Nature Immunol. 11, 148–154 (2010).
Park, S. R. et al. HoxC4 binds to the promoter of the cytidine deaminase AID gene to induce AID expression, class-switch DNA recombination and somatic hypermutation. Nature Immunol. 10, 540–550 (2009).
Dengler, H. S. et al. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nature Immunol. 9, 1388–1398 (2008).
Xu, Z. et al. 14-3-3 adaptor proteins recruit AID to 5′-AGCT-3′-rich switch regions for class switch recombination. Nature Struct. Mol. Biol. 17, 1124–1135 (2010).
Schenten, D. et al. Polζ ablation in B cells impairs the germinal center reaction, class switch recombination, DNA break repair, and genome stability. J. Exp. Med. 206, 477–490 (2009).
Wang, J. H. et al. Mechanisms promoting translocations in editing and switching peripheral B cells. Nature 460, 231–236 (2009).
Jacob, J., Kassir, R. & Kelsoe, G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173, 1165–1175 (1991).
King, I. L. & Mohrs, M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J. Exp. Med. 206, 1001–1007 (2009).
Reinhardt, R. L., Liang, H. E. & Locksley, R. M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature Immunol. 10, 385–393 (2009).
Zaretsky, A. G. et al. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med. 206, 991–999 (2009).
Snapper, C. M. & Paul, W. E. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236, 944–947 (1987).
Cazac, B. B. & Roes, J. TGF-β receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 13, 443–451 (2000).
Mohr, E. et al. IFN-γ produced by CD8 T cells induces T-bet-dependent and -independent class switching in B cells in responses to alum-precipitated protein vaccine. Proc. Natl Acad. Sci. USA 107, 17292–17297 (2010).
Peng, S. L., Szabo, S. J. & Glimcher, L. H. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl Acad. Sci. USA 99, 5545–5550 (2002).
Watanabe, K. et al. Requirement for Runx proteins in IgA class switching acting downstream of TGF-β1 and retinoic acid signaling. J. Immunol. 184, 2785–2792 (2010).
Sellars, M., Reina-San-Martin, B., Kastner, P. & Chan, S. Ikaros controls isotype selection during immunoglobulin class switch recombination. J. Exp. Med. 206, 1073–1087 (2009).
McHeyzer-Williams, L. J. & McHeyzer-Williams, M. G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23, 487–513 (2005).
Toyama, H. et al. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity 17, 329–339 (2002).
Hu, C. C., Dougan, S. K., McGehee, A. M., Love, J. C. & Ploegh, H. L. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. 28, 1624–1636 (2009).
Todd, D. J. et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J. Exp. Med. 206, 2151–2159 (2009).
Gatto, D., Paus, D., Basten, A., Mackay, C. R. & Brink, R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity 31, 259–269 (2009).
Pereira, J. P., Kelly, L. M., Xu, Y. & Cyster, J. G. EBI2 mediates B cell segregation between the outer and centre follicle. Nature 460, 1122–1126 (2009).
Coffey, F., Alabyev, B. & Manser, T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity 30, 599–609 (2009).
Dal Porto, J. M., Haberman, A. M., Kelsoe, G. & Shlomchik, M. J. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J. Exp. Med. 195, 1215–1221 (2002).
Shih, T. A., Meffre, E., Roederer, M. & Nussenzweig, M. C. Role of BCR affinity in T cell dependent antibody responses in vivo. Nature Immunol. 3, 570–575 (2002).
Paus, D. et al. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J. Exp. Med. 203, 1081–1091 (2006).
Schwickert, T. A. et al. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J. Exp. Med. 208, 1243–1252 (2011). This study addresses the early, pre-GC impact of BCR affinity on antigen access and initial priming, which permits cognate contact and regulates GC B cell fate.
Allen, C. D., Okada, T. & Cyster, J. G. Germinal-center organization and cellular dynamics. Immunity 27, 190–202 (2007).
MacLennan, I. C. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994).
Di Noia, J. M. & Neuberger, M. S. Molecular mechanisms of antibody somatic hypermutation. Annu. Rev. Biochem. 76, 1–22 (2007).
Liu, M. et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature 451, 841–845 (2008).
Wang, M., Rada, C. & Neuberger, M. S. Altering the spectrum of immunoglobulin V gene somatic hypermutation by modifying the active site of AID. J. Exp. Med. 207, 141–153 (2010).
Wang, M., Yang, Z., Rada, C. & Neuberger, M. S. AID upmutants isolated using a high-throughput screen highlight the immunity/cancer balance limiting DNA deaminase activity. Nature Struct. Mol. Biol. 16, 769–776 (2009).
Orthwein, A. et al. Regulation of activation-induced deaminase stability and antibody gene diversification by Hsp90. J. Exp. Med. 207, 2751–2765 (2010).
Allen, C. D., Okada, T., Tang, H. L. & Cyster, J. G. Imaging of germinal center selection events during affinity maturation. Science 315, 528–531 (2007).
Hauser, A. E. et al. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity 26, 655–667 (2007).
Schwickert, T. A. et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 446, 83–87 (2007). References 69–71 were seminal studies that used dynamic imaging to observe the movement of GC B cells along follicular DC networks in vivo . Importantly, these authors provided the first evidence of dynamic cognate contact between GC B cells and GC T FH cells in the light zones of active GCs in vivo.
Beltman, J. B., Allen, C. D., Cyster, J. G. & de Boer, R. J. B cells within germinal centers migrate preferentially from dark to light zone. Proc. Natl Acad. Sci. USA 108, 8755–8760 (2011).
Victora, G. D. et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143, 592–605 (2010). A seminal study that provides experimental evidence in vivo for the role of antigen presentation by GC B cells in the selection of affinity-matured memory B cell compartments.
Vikstrom, I. et al. Mcl-1 is essential for germinal center formation and B cell memory. Science 330, 1095–1099 (2010). This study made outstanding use of conditional gene ablation to discriminate between GC and post-GC regulation of antigen-specific B cell survival in vivo.
Vogelzang, A. et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008).
Green, J. A. et al. The sphingosine 1-phosphate receptor S1P2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nature Immunol. 12, 672–680 (2011).
Haynes, N. M. et al. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 179, 5099–5108 (2007).
Good-Jacobson, K. L. et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nature Immunol. 11, 535–542 (2010).
Bhattacharyya, S. et al. NFATc1 affects mouse splenic B cell function by controlling the calcineurin–NFAT signaling network. J. Exp. Med. 208, 823–839 (2011).
Pape, K. A., Taylor, J. J., Maul, R. W., Gearhart, P. J. & Jenkins, M. K. Different B cell populations mediate early and late memory during an endogenous immune response. Science 331, 1203–1207 (2011).
Dogan, I. et al. Multiple layers of B cell memory with different effector functions. Nature Immunol. 10, 1292–1299 (2009).
Kasturi, S. P. et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011).
Phan, T. G. et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. J. Exp. Med. 203, 2419–2424 (2006).
Mohr, E. et al. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J. Immunol. 182, 2113–2123 (2009).
Fooksman, D. R. et al. Development and migration of plasma cells in the mouse lymph node. Immunity 33, 118–127 (2010).
Chevrier, S. et al. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc. Natl Acad. Sci. USA 106, 3895–3900 (2009).
Bernasconi, N. L., Traggiai, E. & Lanzavecchia, A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298, 2199–2202 (2002).
Pelletier, N. et al. Plasma cells negatively regulate the follicular helper T cell program. Nature Immunol. 11, 1110–1118 (2010). This study provided the first evidence that plasma cells retain the capacity for antigen presentation and that this activity can dampen IL-21 and BCL-6 expression in T FH cells.
Maruyama, M., Lam, K. P. & Rajewsky, K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature 407, 636–642 (2000).
Srinivasan, L. et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 139, 573–586 (2009).
Hikida, M. et al. PLC-γ2 is essential for formation and maintenance of memory B cells. J. Exp. Med. 206, 681–689 (2009).
Jelley-Gibbs, D. M. et al. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J. Immunol. 178, 7563–7570 (2007).
Fazilleau, N. et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nature Immunol. 8, 753–761 (2007). This study showed that antigen-specific memory T FH cells remain largely resident in the lymph nodes in which they developed and provided evidence for persistent local antigen presentation.
Liu, W., Meckel, T., Tolar, P., Sohn, H. W. & Pierce, S. K. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity 32, 778–789 (2010). This high resolution imaging study highlights the impact of class-switched BCRs on the dynamics of BCR oligomerization and microclustering. These changes may intrinsically contribute to enhanced memory B cell responsiveness in vivo.
Martin, S. W. & Goodnow, C. C. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nature Immunol. 3, 182–188 (2002).
Engels, N. et al. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nature Immunol. 10, 1018–1025 (2009).
Horikawa, K. et al. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J. Exp. Med. 204, 759–769 (2007).
Waisman, A. et al. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Igα/β. J. Exp. Med. 204, 747–758 (2007).
Wakabayashi, C., Adachi, T., Wienands, J. & Tsubata, T. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science 298, 2392–2395 (2002). References 98 and 99 highlight the enhanced signalling properties of class-switched BCRs, by showing that these BCRs can block the inhibitory action of CD22. Many molecular events may enhance memory B cell function to antigen recall in vivo.
Aiba, Y. et al. Preferential localization of IgG memory B cells adjacent to contracted germinal centers. Proc. Natl Acad. Sci. USA 107, 12192–12197 (2010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- T follicular helper cells
-
(TFH cells). A distinct class of T helper cells specialized to regulate multiple stages of antigen-specific B cell immunity through cognate cell contact and the secretion of cytokines. There are three separable TFH cell subsets defined in the current literature that correspond to the three phases of memory B cell development. These are pre-GC TFH cells, GC TFH cells and memory TFH cells.
- Cognate contact
-
Contact between a B cell and a T follicular helper cell that recognizes the same antigen. This contact requires antigen-receptor engagement by cell-associated antigens or peptide–MHC complexes and can be modified by secondary interactions that can involve both cell-associated and secreted molecules. Cognate contact functions to initiate bidirectional developmental programming.
- Immunoglobulin class switching
-
A region-specific recombination process that occurs in antigen-activated B cells. It occurs between switch-region DNA sequences and results in a change in the class of antibody that is produced — from IgM to either IgG, IgA or IgE. This imparts flexibility to the humoral immune response and allows it to exploit the different capacities of these antibody classes to activate the appropriate downstream effector mechanisms.
- Plasma cells
-
Terminally differentiated, quiescent B cells that develop from plasmablasts and are characterized by the capacity to secrete large amounts of antibodies.
- Germinal centre
-
(GC). A lymphoid structure that arises within lymph node follicles after immunization with, or exposure to, a T cell-dependent antigen. The GC is specialized for facilitating the development of high-affinity, long-lived plasma cells and memory B cells.
- GC reaction
-
(Germinal centre reaction). A cycle of activity characterized by three stages. First, GC B cells undergo clonal expansion and B cell receptor diversification in the GC dark zone. The B cells then scan follicular dendritic cells for antigens, and finally make contact with cognate GC TFH cells in the GC light zone. Positive selection continues the GC cycle with re-entry into the dark zone or promotes exit from the GC into the memory B cell compartment.
- Class-specific memory B cells
-
Non-secreting memory B cells that express either IgM or downstream non-IgM antibody classes following T helper cell-regulated class-switch recombination.
- Subcapsular sinus macrophages
-
(SCS macrophages). A CD11b+CD169+ macrophage subset that populates the subcapsular sinus region of lymph nodes. These cells function to trap particulate antigens from the lymph and present antigens to follicular B cells.
- Follicular DCs
-
(Follicular dendritic cells). Specialized non-haematopoietic stromal cells that reside in the lymphoid follicles and germinal centres. These cells possess long dendrites and carry intact antigens on their surface. They are crucial for the optimal selection of B cells that produce antigen-binding antibodies.
- Activation-induced cytidine deaminase
-
(AID). An enzyme that is required for two crucial events in the germinal centre: somatic hypermutation and class-switch recombination.
- Non-homologous end joining
-
(NHEJ). A mechanism for repairing double-strand DNA breaks that does not require homologous sequences for ligation. NHEJ is used to complete recombination during antibody class switching.
- Somatic hypermutation
-
A process in which point mutations are generated in the immunoglobulin variable-region gene segments of cycling centroblasts. Some mutations might generate a binding site with increased affinity for the specific antigen, but others can lead to loss of antigen recognition by the B cell receptor or the generation of a self-reactive B cell receptor.
- Unfolded-protein response
-
A response that increases the ability of the endoplasmic reticulum to fold and translocate proteins, decreases the synthesis of proteins, and can cause cell cycle arrest and apoptosis.
Rights and permissions
About this article
Cite this article
McHeyzer-Williams, M., Okitsu, S., Wang, N. et al. Molecular programming of B cell memory. Nat Rev Immunol 12, 24–34 (2012). https://doi.org/10.1038/nri3128
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3128
This article is cited by
-
B cell-specific knockout of AID protects against atherosclerosis
Scientific Reports (2023)
-
RNA sequencing of blood from sex- and age-matched discordant siblings supports immune and transcriptional dysregulation in autism spectrum disorder
Scientific Reports (2023)
-
Bioengineering translational models of lymphoid tissues
Nature Reviews Bioengineering (2023)
-
Identifying Key Genes and Functionally Enriched Pathways in Osteoporotic Patients by Weighted Gene Co-Expression Network Analysis
Biochemical Genetics (2023)
-
Infection pre-Ad26.COV2.S-vaccination primes greater class switching and reduced CXCR5 expression by SARS-CoV-2-specific memory B cells
npj Vaccines (2023)