Key Points
-
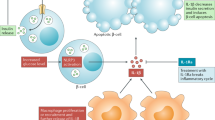
Type 2 diabetes is associated with obesity, ageing and inactivity. It is due to a progressive failure of pancreatic islet β-cells to compensate for insulin resistance.
-
The proposed mechanisms to explain impaired insulin secretion and sensitivity in type 2 diabetes include oxidative stress, endoplasmic reticulum stress, amyloid deposition in the pancreas, ectopic lipid deposition in muscle, liver and pancreas, and lipotoxicity and glucotoxicity. All these cellular stresses may induce an inflammatory response or are exacerbated by or associated with inflammation.
-
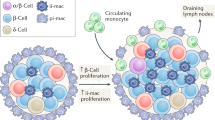
Factors that are associated with innate immune responses are present in the circulation, insulin-sensitive tissues and pancreatic islets in type 2 diabetes, and this evidence supports the involvement of inflammation in the pathogenesis of this disease.
-
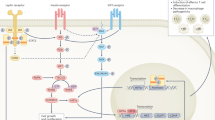
Mechanisms thought to be responsible for the inflammatory state in type 2 diabetes include hypoxia and cell death of expanding adipose tissue, activation of the nuclear factor-κB (NF-κB) and JUN N-terminal kinase (JNK) pathways, activation of interleukin-1β (IL-1β), and recruitment and activation of immune cells.
-
Clinical trials using IL-1 antagonists or salsalate to directly target pro-inflammatory factors in patients with type 2 diabetes show promising preliminary results and support the role of inflammation in this condition.
-
Existing data suggest a potential role for inflammation in the pathogenesis of type 2 diabetes. The relative importance of this mechanism and the precise therapeutic consequences remain to be elucidated.
Abstract
Components of the immune system are altered in obesity and type 2 diabetes (T2D), with the most apparent changes occurring in adipose tissue, the liver, pancreatic islets, the vasculature and circulating leukocytes. These immunological changes include altered levels of specific cytokines and chemokines, changes in the number and activation state of various leukocyte populations and increased apoptosis and tissue fibrosis. Together, these changes suggest that inflammation participates in the pathogenesis of T2D. Preliminary results from clinical trials with salicylates and interleukin-1 antagonists support this notion and have opened the door for immunomodulatory strategies for the treatment of T2D that simultaneously lower blood glucose levels and potentially reduce the severity and prevalence of the associated complications of this disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Shoelson, S. E., Lee, J. & Goldfine, A. B. Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006).
Donath, M. Y., Boni-Schnetzler, M., Ellingsgaard, H. & Ehses, J. A. Islet inflammation impairs the pancreatic β-cell in type 2 diabetes. Physiology 24, 325–331 (2009).
Bonner-Weir, S. Islet growth and development in the adult. J. Mol. Endocrinol. 24, 297–302 (2000).
Kahn, B. B. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell 92, 593–596 (1998).
Rhodes, C. J. Type 2 diabetes-a matter of β-cell life and death? Science 307, 380–384 (2005).
Robertson, R. P., Harmon, J., Tran, P. O. & Poitout, V. β-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 53, S119–S124 (2004).
Weir, G. C. & Bonner-Weir, S. Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 53, S16–S21 (2004).
Prentki, M. & Nolan, C. J. Islet β cell failure in type 2 diabetes. J. Clin. Invest. 116, 1802–1812 (2006).
Hull, R. L., Westermark, G. T., Westermark, P. & Kahn, S. E. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 89, 3629–3643 (2004).
Harding, H. P. & Ron, D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes 51, S455–S461 (2002).
Hotamisligil, G. S. & Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nature Rev. Immunol. 8, 923–934 (2008).
Donath, M. Y., Storling, J., Maedler, K. & Mandrup-Poulsen, T. Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. J. Mol. Med. 81, 455–470 (2003).
Ehses, J. A., Ellingsgaard, H., Boni-Schnetzler, M. & Donath, M. Y. Pancreatic islet inflammation in type 2 diabetes: from α and β cell compensation to dysfunction. Arch. Physiol. Biochem. 115, 240–247 (2009).
Donath, M. Y. et al. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 31, S161–S164 (2008).
Masters, S. L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nature Immunol. 11, 897–904 (2010).
Pickup, J. C., Mattock, M. B., Chusney, G. D. & Burt, D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40, 1286–1292 (1997).
Spranger, J. et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European prospective investigation into cancer and nutrition (EPIC)-potsdam study. Diabetes 52, 812–817 (2003).
Herder, C. et al. Inflammation and type 2 diabetes: results from KORA Augsburg. Gesundheitswesen 67, S115–S121 (2005).
Herder, C. et al. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care 32, 421–423 (2009).
Pradhan, A. D., Manson, J. E., Rifai, N., Buring, J. E. & Ridker, P. M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286, 327–334 (2001).
Meier, C. A. et al. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J. Clin. Endocrinol. Metab. 87, 1184–1188 (2002).
Carstensen, M. et al. Accelerated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: Whitehall II prospective cohort study. Diabetes 59, 1222–1227 (2010).
Marculescu, R. et al. Interleukin-1 receptor antagonist genotype is associated with coronary atherosclerosis in patients with type 2 diabetes. Diabetes 51, 3582–3585 (2002).
Dinarello, C. A. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N. Engl. J. Med. 343, 732–734 (2000).
Larsen, C. M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007). A proof-of-concept clinical study demonstrating the potential of immunomodulation with an IL-1 antagonist in T2D.
Ehses, J. A. et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc. Natl Acad. Sci. USA 106, 13998–14003 (2009).
Donath, M. Y. et al. XOMA 052, an anti-IL-1β antibody, in a double-blind, placebo-controlled, dose escalation study of the safety and pharmacokinetics in patients with type 2 diabetes mellitus – a new approach to therapy. Diabetologia 51, S7 (2008).
Goldfine, A. B. et al. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann. Intern. Med. 152, 346–357 (2010). This clinical trial showed that salsalate improves circulating glucose and lipid levels in patients with T2D.
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993). This early study suggested that TNF could cause insulin resistance.
Schreyer, S. A., Chua, S. C. Jr & LeBoeuf, R. C. Obesity and diabetes in TNF-α receptor- deficient mice. J. Clin. Invest. 102, 402–411 (1998).
Bernstein, L. E., Berry, J., Kim, S., Canavan, B. & Grinspoon, S. K. Effects of etanercept in patients with the metabolic syndrome. Arch. Intern. Med. 166, 902–908 (2006).
Dominguez, H. et al. Metabolic and vascular effects of tumor necrosis factor-α blockade with etanercept in obese patients with type 2 diabetes. J. Vasc. Res. 42, 517–525 (2005).
Lo, J. et al. Effects of TNF-α neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 293, E102–E109 (2007).
Ofei, F., Hurel, S., Newkirk, J., Sopwith, M. & Taylor, R. Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45, 881–885 (1996).
Paquot, N., Castillo, M. J., Lefebvre, P. J. & Scheen, A. J. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J. Clin. Endocrinol. Metab. 85, 1316–1319 (2000).
Rosenvinge, A., Krogh-Madsen, R., Baslund, B. & Pedersen, B. K. Insulin resistance in patients with rheumatoid arthritis: effect of anti-TNFα therapy. Scand. J. Rheumatol. 36, 91–96 (2007).
Kiortsis, D. N., Mavridis, A. K., Vasakos, S., Nikas, S. N. & Drosos, A. A. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann. Rheum. Dis. 64, 765–766 (2005).
Stanley, T. L. et al. TNF-α antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J. Clin. Endocrinol. Metab. 3 Nov 2010 (doi:10.1210/jc.2010-1170).
Yazdani-Biuki, B. et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-α antibody infliximab: a case observation. Diabetes Care 29, 1712–1713 (2006).
Yazdani-Biuki, B. et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur. J. Clin. Invest. 34, 641–642 (2004).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003). References 41 and 42 identified macrophages in adipose tissue and showed that their numbers increased with obesity.
Lumeng, C. N., Deyoung, S. M., Bodzin, J. L. & Saltiel, A. R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56, 16–23 (2007).
Shaul, M. E., Bennett, G., Strissel, K. J., Greenberg, A. S. & Obin, M. S. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet–induced obesity in mice. Diabetes 59, 1171–1181 (2010).
Kosteli, A. et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Invest. 120, 3466–3479 (2010).
Herrero, L., Shapiro, H., Nayer, A., Lee, J. & Shoelson, S. E. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc. Natl Acad. Sci. USA 107, 240–245 (2010).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Liu, J. et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nature Med. 15, 940–945 (2009).
Wu, H. et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115, 1029–1038 (2007).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature Med. 15, 930–939 (2009).
Ilan, Y. et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc. Natl Acad. Sci. USA 107, 9765–9770 (2010).
Baecher-Allan, C. & Hafler, D. A. Human regulatory T cells and their role in autoimmune disease. Immunol. Rev. 212, 203–216 (2006).
Roncarolo, M. G. & Battaglia, M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nature Rev. Immunol. 7, 585–598 (2007).
Maedler, K. et al. Leptin modulates β cell expression of IL-1 receptor antagonist and release of IL-1β in human islets. Proc. Natl Acad. Sci. USA 101, 8138–8143 (2004).
Maedler, K. et al. Glucose-induced β-cell production of interleukin-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 110, 851–860 (2002). The first description of the role of IL-1β in T2D.
Ehses, J. A. et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 (2007). The original description of macrophage infiltration in islets of patients with T2D.
Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005).
Hosogai, N. et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56, 901–911 (2007).
Yin, J. et al. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 296, E333–E342 (2009).
Pasarica, M. et al. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J. Clin. Endocrinol. Metab. 95, 4052–4055 (2010).
Pasarica, M. et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 (2009).
Murdoch, C., Muthana, M. & Lewis, C. E. Hypoxia regulates macrophage functions in inflammation. J. Immunol. 175, 6257–6263 (2005).
Burke, B. et al. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am. J. Pathol. 163, 1233–1243 (2003).
Cinti, S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 (2005).
Strissel, K. J. et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56, 2910–2918 (2007).
Arkan, M. C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nature Med. 11, 191–198 (2005).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nature Med. 11, 183–190 (2005).
Solinas, G. & Karin, M. JNK1 and IKKβ: molecular links between obesity and metabolic dysfunction. FASEB J. 24, 2596–2611 (2010).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Sabio, G. et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 (2008).
Solinas, G. et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell. Metab. 6, 386–397 (2007).
Tuncman, G. et al. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc. Natl Acad. Sci. USA 103, 10741–10746 (2006).
Cai, D. et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119, 285–298 (2004).
Zhang, X. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Eldor, R. et al. Conditional and specific NF-κB blockade protects pancreatic β cells from diabetogenic agents. Proc. Natl Acad. Sci. USA 103, 5072–5077 (2006).
Mooney, R. A. Counterpoint: interleukin-6 does not have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 102, 816–818 (2007).
Pedersen, B. K. & Febbraio, M. A. Point: interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 102, 814–816 (2007).
Fried, S. K., Bunkin, D. A. & Greenberg, A. S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 83, 847–850 (1998).
Wunderlich, F. T. et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell. Metab. 12, 237–249 (2010).
Matthews, V. B. et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53, 2431–2441 (2010).
Donath, M. Y., Gross, D. J., Cerasi, E. & Kaiser, N. Hyperglycemia-induced β-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes 48, 738–744 (1999).
Maedler, K. et al. Glucose induces β-cell apoptosis via upregulation of the Fas-receptor in human islets. Diabetes 50, 1683–1690 (2001).
Schumann, D. M. et al. The Fas pathway is involved in pancreatic β cell secretory function. Proc. Natl Acad. Sci. USA 104, 2861–2866 (2007).
Boni-Schnetzler, M. et al. Increased interleukin (IL)-1β messenger ribonucleic acid expression in β-cells of individuals with type 2 diabetes and regulation of IL-1β in human islets by glucose and autostimulation. J. Clin. Endocrinol. Metab. 93, 4065–4074 (2008).
Boni-Schnetzler, M. et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150, 5218–5229 (2009).
Ehses, J. A. et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and β cell dysfunction induced by a high-fat diet. Diabetologia 53, 1795–1806 (2010).
Haversen, L., Danielsson, K. N., Fogelstrand, L. & Wiklund, O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 202, 382–393 (2009).
Lee, J. Y., Sohn, K. H., Rhee, S. H. & Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 276, 16683–16689 (2001).
Lee, J. Y. et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J. Biol. Chem. 279, 16971–16979 (2004).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Immunol. 11, 136–140 (2010). A study that describes the mechanism of glucose-induced IL-1β production in pancreatic islets.
van de Veerdonk, F. L. et al. Reactive oxygen species-independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl Acad. Sci. USA 107, 3030–3033 (2010).
Meissner, F. et al. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 116, 1570–1573 (2010).
Schroder, K., Zhou, R. & Tschopp, J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010).
Dinarello, C. A. Biologic basis for interleukin-1 in disease. Blood 87, 2095–2147 (1996).
Ehses, J. A. et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc. Natl Acad. Sci. USA 106, 13998–14003 (2009).
Bruun, J. M., Lihn, A. S., Pedersen, S. B. & Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 90, 2282–2289 (2005).
Harman-Boehm, I. et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 92, 2240–2247 (2007).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006).
Sartipy, P. & Loskutoff, D. J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl Acad. Sci. USA 100, 7265–7270 (2003).
Kamei, N. et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 281, 26602–26614 (2006).
Jiao, P. et al. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-κB and c-Jun NH2-terminal kinase pathways. Diabetes 58, 104–115 (2009).
Ehses, J. A. et al. IL-1β-MyD88 signaling is central to islet chemokine secretion in response to metabolic stress: evidence from a spontaneous model of type 2 diabetes, the GK rat. Diabetologia 50 S177 (2007).
Marselli, L. et al. Evidence of inflammatory markers in β cells of type 2 diabetic subjects. Diabetologia 50, S178–S179 (2007).
Wolf, A. M., Wolf, D., Rumpold, H., Enrich, B. & Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 323, 630–635 (2004).
Greenstein, A. S. et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119, 1661–1670 (2009).
Rutkowski, J. M., Davis, K. E. & Scherer, P. E. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 276, 5738–5746 (2009).
Fleischman, A., Shoelson, S. E., Bernier, R. & Goldfine, A. B. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care 31, 289–294 (2008).
Larsen, C. M. et al. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care 32, 1663–1668 (2009).
Gonzalez-Gay, M. A. et al. Anti-tumor necrosis factor-α blockade improves insulin resistance in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 24, 83–86 (2006).
Gonzalez-Gay, M. A. et al. Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-α therapy. Ann. NY Acad. Sci. 1193, 153–159 (2010).
Huvers, F. C., Popa, C., Netea, M. G., van den Hoogen, F. H. & Tack, C. J. Improved insulin sensitivity by anti-TNFα antibody treatment in patients with rheumatic diseases. Ann. Rheum. Dis. 66, 558–559 (2007).
Yuan, M. et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKKβ. Science 293, 1673–1677 (2001). This study initially identified a potential role for NF-κB in T2D and showed that salicylates improved blood glucose levels in rodent models.
Frantz, B. & O'Neill, E. A. The effect of sodium salicylate and aspirin on NF-κB. Science 270, 2017–2019 (1995).
Jurivich, D. A., Sistonen, L., Kroes, R. A. & Morimoto, R. I. Effect of sodium salicylate on the human heat shock response. Science 255, 1243–1245 (1992).
Hundal, R. S. et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Invest. 109, 1321–1326 (2002).
Bonner-Weir, S., Trent, D. F. & Weir, G. C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J. Clin. Invest. 71, 1544–1553 (1983).
Leahy, J. L., Cooper, H. E., Deal, D. A. & Weir, G. C. Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J. Clin. Invest. 77, 908–915 (1986).
Yki-Jarvinen, H., Helve, E. & Koivisto, V. A. Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes 36, 892–896 (1987).
Rossetti, L., Smith, D., Shulman, G. I., Papachristou, D. & DeFronzo, R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J. Clin. Invest. 79, 1510–1515 (1987).
Reaven, G. M., Hollenbeck, C., Jeng, C. Y., Wu, M. S. & Chen, Y. D. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37, 1020–1024 (1988).
Walker, K. Z. et al. Body fat distribution and non-insulin-dependent diabetes: comparison of a fiber-rich, high-carbohydrate, low-fat (23%) diet and a 35% fat diet high in monounsaturated fat. Am. J. Clin. Nutr. 63, 254–260 (1996).
Maedler, K., Oberholzer, J., Bucher, P., Spinas, G. A. & Donath, M. Y. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic β-cell turnover and function. Diabetes 52, 726–733 (2003).
Maedler, K. et al. Distinct effects of saturated and monounsaturated fatty acids on β-cell turnover and function. Diabetes 50, 69–76 (2001).
Unger, R. H. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 44, 863–870 (1995).
Prentki, M. & Corkey, B. E. Are the β-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes 45, 273–283 (1996).
Unger, R. H., Clark, G. O., Scherer, P. E. & Orci, L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta 1801, 209–214 (2010).
Poitout, V. & Robertson, R. P. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr. Rev. 29, 351–366 (2008).
Evans, J. L., Goldfine, I. D., Maddux, B. A. & Grodsky, G. M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 23, 599–622 (2002).
Evans, J. L., Goldfine, I. D., Maddux, B. A. & Grodsky, G. M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 52, 1–8 (2003).
Araki, E., Oyadomari, S. & Mori, M. Endoplasmic reticulum stress and diabetes mellitus. Intern. Med. 42, 7–14 (2003).
Izumi, T. et al. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes 52, 409–416 (2003).
Hotamisligil, G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010).
Zraika, S. et al. Toxic oligomers and islet β cell death: guilty by association or convicted by circumstantial evidence? Diabetologia 53, 1046–1056 (2010).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 33, S62–S69 (2010).
Kibirige, M., Metcalf, B., Renuka, R. & Wilkin, T. J. Testing the accelerator hypothesis: the relationship between body mass and age at diagnosis of type 1 diabetes. Diabetes Care 26, 2865–2870 (2003).
Wilkin, T. J. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia 44, 914–922 (2001).
Donath, M. Y. & Halban, P. A. Decreased β-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia 47, 581–589 (2004).
Hypponen, E., Virtanen, S. M., Kenward, M. G., Knip, M. & Akerblom, H. K. Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care 23, 1755–1760 (2000).
Libman, I. M., Pietropaolo, M., Arslanian, S. A., LaPorte, R. E. & Becker, D. J. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care 26, 2871–2875 (2003).
Fourlanos, S., Narendran, P., Byrnes, G. B., Colman, P. G. & Harrison, L. C. Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 47, 1661–1667 (2004).
Nathan, D. M. et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 32, 193–203 (2009).
Donath, M. Y. & Ehses, J. A. Type 1, type 1.5, and type 2 diabetes: NOD the diabetes we thought it was. Proc. Natl Acad. Sci. USA 103, 12217–12218 (2006).
Goldfine, A. B. et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin. Transl. Sci. 1, 36–43 (2008).
Koska, J. et al. The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised double-blind placebo-controlled study. Diabetologia 52, 385–393 (2009).
Acknowledgements
The authors wish to thank their scientific collaborators who have contributed so much to these studies, in particular A. Goldfine, J. Lee, D. Mathis, K. Maedler, P. Halban, T. Mandrup-Poulsen, J. Ehses and M. Boni-Schnetzler.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Marc Y. Donath is listed as the inventor of a patent filed in 2003 for the use of an interleukin-1 receptor antagonist for the treatment of or prophylaxis against type 2 diabetes. He is a consultant for Novartis, XOMA, Eli Lilly and Company, Cytos, Merck and AstraZeneca. Steven E. Shoelson holds patents on the use of salicylates in diabetes, prediabetes and cardiovascular disease. He has consulted for Catabasis, Amylin, AstraZeneca, Merck, Genentech, XOMA and Kowa.
Related links
Glossary
- Insulin resistance
-
A pathological condition in which insulin becomes less effective at lowering blood glucose levels.
- Endoplasmic reticulum stress
-
(ER stress). A response by the ER that results in the disruption of protein folding and the accumulation of unfolded proteins in the ER.
- Lipotoxicity
-
The toxic effects of elevated levels of free fatty acids. These detrimental effects may be functional and reversible, or may lead to cell death.
- Glucotoxicity
-
The toxic effects of hyperglycaemia. These detrimental effects may be functional and reversible, or may lead to cell death.
- Autoinflammatory disease
-
A disease resulting from an attack by the innate immune system on the body's own tissues. By contrast, autoimmune diseases are caused by the pathological activation of adaptive immune responses. Autoimmune and autoinflammatory diseases have some characteristics in common, including shared effector mechanisms.
- M1-type macrophage
-
A macrophage that is activated by Toll-like receptor ligands (such as lipopolysaccharide) and interferon-γ, and that expresses inducible nitric oxide synthase, which generates nitric oxide.
- M2-type macrophage
-
A macrophage that is stimulated by interleukin-4 (IL-4) or IL-13 and that expresses arginase 1, the mannose receptor CD206 and the IL-4 receptor α-chain.
- KitW–sh/W–sh mice
-
The KitW–sh (or sash) mutation abolishes KIT expression in mast cells, and the mutant mice are deficient in mast cells.
- Insulitis
-
Inflammation of the pancreatic islets during the progression of diabetes. Insulitis in type 1 diabetes is caused by autoimmunity and in type 2 diabetes by metabolic stressors such as hyperglycaemia and elevated levels of free fatty acids.
- Ischaemia
-
A condition in which the flow of blood to a tissue or organs is less than normal, and which results in injury to that tissue or organ.
- Cachexia
-
Severe weight loss, muscle wasting and debility caused by prolonged disease. It is thought to be mediated through neuroimmunoendocrine interactions.
- Leptin
-
A protein hormone that regulates energy intake and expenditure. It is one of the most important adipose-derived hormones and its production correlates with the mass of adipose tissue.
- Inflammasome
-
A molecular complex of several proteins that, when activated, results in the production of active caspase 1, which cleaves pro-interleukin-1β (pro-IL-1β) and pro-IL-18 to produce the active cytokines.
- Salsalate
-
A prodrug form of salicylic acid that has fewer side effects than sodium salicylate. Salsalate is approved for use in humans as a source of salicylic acid.
Rights and permissions
About this article
Cite this article
Donath, M., Shoelson, S. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11, 98–107 (2011). https://doi.org/10.1038/nri2925
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2925
This article is cited by
-
The effects of suppressing inflammation by tofacitinib may simultaneously improve glycaemic parameters and inflammatory markers in rheumatoid arthritis patients with comorbid type 2 diabetes: a proof-of-concept, open, prospective, clinical study
Arthritis Research & Therapy (2024)
-
The interaction of perfluoroalkyl acids and a family history of diabetes on arthritis: analyses of 2011–2018 NHANES
BMC Public Health (2024)
-
Diabetes-induced male infertility: potential mechanisms and treatment options
Molecular Medicine (2024)
-
Measurement of cumulative high-sensitivity C-reactive protein and monocyte to high-density lipoprotein ratio in the risk prediction of type 2 diabetes: a prospective cohort study
Journal of Translational Medicine (2024)
-
The role of regulated necrosis in diabetes and its complications
Journal of Molecular Medicine (2024)