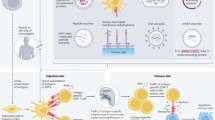
Key Points
-
Recent US Food and Drug Administration (FDA) approval of the prostate cancer vaccine known as sipuleucel-T (Provenge; Dendreon Inc) marks the first antigen-specific immunotherapy approved for the treatment of cancer.
-
Several other antigen-specific immunotherapy approaches are under development for cancer treatment. Examples include those based on poxviral and DNA vaccines, as well as radioisotope-labelled monoclonal antibodies directed at a specific tumour antigen.
-
Approaches to cancer immunotherapy that are not directed at a particular antigen include cell-based vaccines, as well as the blockade of immunological checkpoints mediated by CTLA4 and PD1 using monoclonal antibodies.
-
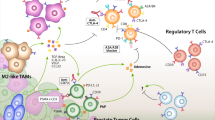
Prostate and other tumours probably arise in the context of pre-existing inflammation which could be actively contributing to tumour progression.
-
Conventional treatments for prostate cancer, such as androgen ablation, have immunological effects that could prove beneficial in terms of supporting an antitumour immune response.
Abstract
Advances in basic immunology have led to an improved understanding of the interactions between the immune system and tumours, generating renewed interest in approaches that aim to treat cancer immunologically. As clinical and preclinical studies of tumour immunotherapy illustrate several immunological principles, a review of these data is broadly instructive and is particularly timely now that several agents are beginning to show evidence of efficacy. This is especially relevant in the case of prostate cancer, as recent approval of sipuleucel-T by the US Food and Drug Administration marks the first antigen-specific immunotherapy approved for cancer treatment. Although this Review focuses on immunotherapy for prostate cancer, the principles discussed are applicable to many tumour types, and the approaches discussed are highlighted in that context.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
[No authors listed]. Stat. bite: estimated worldwide cancer mortality among men, 2002. J. Natl Cancer Inst. 97, 1402 (2005).
Walsh, P. C., DeWeese, T. L. & Eisenberger, M. A. Clinical practice. Localized prostate cancer. N. Engl. J. Med. 357, 2696–2705 (2007).
Carter, H. B. et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J. Urol. 178, 2359–2364 (2007).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Petrylak, D. P. et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351, 1513–1520 (2004).
Sun, J. et al. Cumulative effect of five genetic variants on prostate cancer risk in multiple study populations. Prostate 68, 1257–1262 (2008).
Kolonel, L. N., Altshuler, D. & Henderson, B. E. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nature Rev. Cancer 4, 519–527 (2004).
De Marzo, A. M. et al. Inflammation in prostate carcinogenesis. Nature Rev. Cancer 7, 256–269 (2007).
Ammirante, M., Luo, J. L., Grivennikov, S., Nedospasov, S. & Karin, M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305 (2010). A recent study supporting the concept that B cells mediate the progression of prostate cancer from an androgen-dependent to a castration-resistant state in a mouse model.
Luo, J. L. et al. Nuclear cytokine-activated IKKα controls prostate cancer metastasis by repressing Maspin. Nature 446, 690–694 (2007).
de Visser, K. E., Eichten, A. & Coussens, L. M. Paradoxical roles of the immune system during cancer development. Nature Rev. Cancer 6, 24–37 (2006).
Sfanos, K. S. et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin. Cancer Res. 14, 3254–3261 (2008).
Miller, A. M. et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J. Immunol. 177, 7398–7405 (2006).
Fox, S. B. et al. The number of regulatory T cells in prostate cancer is associated with the androgen receptor and hypoxia-inducible factor (HIF)-2α but not HIF-1α. Prostate 67, 623–629 (2007).
Kiniwa, Y. et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin. Cancer Res. 13, 6947–6958 (2007).
Bronte, V. et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 201, 1257–1268 (2005).
Zippelius, A. et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 64, 2865–2873 (2004).
Scher, H. I. & Heller, G. Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology 55, 323–327 (2000).
Pound, C. R. et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281, 1591–1597 (1999).
Sandler, H. M. & Eisenberger, M. A. Assessing and treating patients with increasing prostate specific antigen following radical prostatectomy. J. Urol. 178, S20–S24 (2007).
Freedland, S. J. & Moul, J. W. Prostate specific antigen recurrence after definitive therapy. J. Urol. 177, 1985–1991 (2007).
Locker, G. Y. et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 24, 5313–5327 (2006).
Drake, C. G., Jaffee, E. & Pardoll, D. M. Mechanisms of immune evasion by tumors. Adv. Immunol. 90, 51–81 (2006).
Denmeade, S. R. & Isaacs, J. T. A history of prostate cancer treatment. Nature Rev. Cancer 2, 389–396 (2002).
Aragon-Ching, J. B., Williams, K. M. & Gulley, J. L. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front. Biosci. 12, 4957–4971 (2007).
Mercader, M. et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc. Natl Acad. Sci. USA 98, 14565–14570 (2001). The first clinical demonstration that androgen ablation alters the local immune environment in the prostate gland, resulting in an influx of CD4+ T cells with effector function.
Gannon, P. O. et al. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J. Immunol. Methods 348, 9–17 (2009).
Drake, C. G. et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 7, 239–249 (2005). A study in mice with autochronous prostate tumours supporting the concept that androgen ablation results in a mitigation of specfic CD4+ T cell tolerance to the prostate gland.
Sutherland, J. S. et al. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 175, 2741–2753 (2005).
Arlen, P. M. et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J. Urol. 174, 539–546 (2005).
Koh, Y. T., Gray, A., Higgins, S. A., Hubby, B. & Kast, W. M. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate 69, 571–584 (2009).
Adler, H. L. et al. Elevated levels of circulating interleukin-6 and transforming growth factor-β1 in patients with metastatic prostatic carcinoma. J. Urol. 161, 182–187 (1999).
Willimsky, G. & Blankenstein, T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature 437, 141–146 (2005).
Mihalyo, M. A., Hagymasi, A. T., Slaiby, A. M., Nevius, E. E. & Adler, A. J. Dendritic cells program non-immunogenic prostate-specific T cell responses beginning at early stages of prostate tumorigenesis. Prostate 67, 536–546 (2007).
Greenberg, N. M. et al. Prostate cancer in a transgenic mouse. Proc. Natl Acad. Sci. USA 92, 3439–3443 (1995).
Degl'Innocenti, E. et al. Peripheral T cell tolerance occurs early during spontaneous prostate cancer development and can be rescued by dendritic cell immunization. Eur. J. Immunol. 35, 66–75 (2005).
Bai, A., Higham, E., Eisen, H. N., Wittrup, K. D. & Chen, J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc. Natl Acad. Sci. USA 105, 13003–13008 (2008).
Anderson, M. J., Shafer-Weaver, K., Greenberg, N. M. & Hurwitz, A. A. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J. Immunol. 178, 1268–1276 (2007).
Lees, J. R. et al. T-cell recognition of a prostate specific antigen is not sufficient to induce prostate tissue destruction. Prostate 66, 578–590 (2006).
Grosso, J. F. et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 117, 3383–3392 (2007). These data show that lymphocyte activation gene 3 (LAG3) is an important immune checkpoint on CD8+ T cells and that blocking LAG3 can enhance T cell effector function in mice with cancer.
Wada, S. et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 69, 4309–4318 (2009).
Speiser, D. E. et al. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J. Exp. Med. 186, 645–653 (1997).
Lyman, M. A., Aung, S., Biggs, J. A. & Sherman, L. A. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J. Immunol. 172, 6558–6567 (2004).
Getnet, D. et al. Tumor recognition and self-recognition induce distinct transcriptional profiles in antigen-specific CD4 T cells. J. Immunol. 182, 4675–4685 (2009).
Shafer-Weaver, K. A. et al. Cutting edge: tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J. Immunol. 183, 4848–4852 (2009).
Milowsky, M. I. et al. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J. Clin. Oncol. 25, 540–547 (2007).
Williams, S. A., Singh, P., Isaacs, J. T. & Denmeade, S. R. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate 67, 312–329 (2007).
Fong, L. et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 69, 609–615 (2009).
Arlen, P. M., Kaufman, H. L. & DiPaola, R. S. Pox viral vaccine approaches. Semin. Oncol. 32, 549–555 (2005).
Hodge, J. W. et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 59, 5800–5807 (1999).
Terasawa, H., Tsang, K. Y., Gulley, J., Arlen, P. & Schlom, J. Identification and characterization of a human agonist cytotoxic T-lymphocyte epitope of human prostate-specific antigen. Clin. Cancer Res. 8, 41–53 (2002).
Harrington, L. E., Most, R. R., Whitton, J. L. & Ahmed, R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 76, 3329–3337 (2002).
Kaufman, H. L. et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 22, 2122–2132 (2004).
Madan, R. A., Arlen, P. M., Mohebtash, M., Hodge, J. W. & Gulley, J. L. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin. Investig. Drugs 18, 1001–1011 (2009).
Gulley, J. L., Madan, R. A. & Arlen, P. M. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine 25, B89–B96 (2007).
Higano, C. S. et al. Integrated data from 2 randomized, double-blind, placebo-controlled, Phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115, 3670–3679 (2009).
Gilboa, E. DC-based cancer vaccines. J. Clin. Invest. 117, 1195–1203 (2007).
Fong, L., Ruegg, C. L., Brockstedt, D., Engleman, E. G. & Laus, R. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J. Immunol. 159, 3113–3117 (1997).
Rice, J., Ottensmeier, C. H. & Stevenson, F. K. DNA vaccines: precision tools for activating effective immunity against cancer. Nature Rev. Cancer 8, 108–120 (2008).
Tsen, S. W., Paik, A. H., Hung, C. F. & Wu, T. C. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Rev. Vaccines 6, 227–239 (2007).
Shi, Y. et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 16, 126–133 (2006).
McNeel, D. G. et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase (PAP) in patients with stage D0 prostate cancer. J. Clin. Oncol. 27, 425–430 (2009). A recent Phase I trial showing how DNA-based vaccines can be used in prostate cancer. This trial is noteworthy because it targeted a population of patients with early-stage disease and because the DNA vector used is flexible in terms of potential antigens that could be targeted.
Tagawa, S. T. et al. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer 116, 1075–1083 (2010).
Nanus, D. M. et al. Clinical use of monoclonal antibody HuJ591 therapy: targeting prostate specific membrane antigen. J. Urol. 170, S84–S88 (2003).
Bander, N. H. et al. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J. Clin. Oncol. 23, 4591–4601 (2005).
Keilholz, U. et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the society for biological therapy. J. Immunother. 25, 97–138 (2002).
Jager, E. et al. Immunoselection in vivo: independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int. J. Cancer 71, 142–147 (1997).
Ohnmacht, G. A. et al. Short-term kinetics of tumor antigen expression in response to vaccination. J. Immunol. 167, 1809–1820 (2001).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993). This study provided the initial immunological and scientific rationale for targeting cancer using tumour cell vaccines engineered to secrete GM-CSF.
Simons, J. W. & Sacks, N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol. Oncol. 24, 419–424 (2006).
Simons, J. W. et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 57, 1537–1546 (1997).
Thomas, A. M. et al. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J. Exp. Med. 200, 297–306 (2004).
Jaffee, E. M. et al. Novel allogeneic granulocyte–macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a Phase I trial of safety and immune activation. J. Clin. Oncol. 19, 145–156 (2001).
Emens, L. A. et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J. Clin. Oncol. 27, 5911–5918 (2009).
Nemunaitis, J. et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 13, 555–562 (2006).
Borrello, I. M. et al. Granulocyte–macrophage colony-stimulating factor (GM-CSF)-secreting cellular immunotherapy in combination with autologous stem cell transplantation (ASCT) as postremission therapy for acute myeloid leukemia (AML). Blood 114, 1736–1745 (2009).
Small, E. J. et al. Granulocyte macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 13, 3883–3891 (2007).
Nguyen, M. C. et al. Antibody responses to galectin-8, TARP and TRAP1 in prostate cancer patients treated with a GM-CSF-secreting cellular immunotherapy. Cancer Immunol. Immunother. 59, 1313–1323 (2010).
Higano, C. et al. A Phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC) [online]. American Society of Clinical Oncology 2009 Genitourinary Cancers Symposium (2009).
Fong, L. & Small, E. J. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J. Clin. Oncol. 26, 5275–5283 (2008).
Chen, L. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nature Rev. Immunol. 4, 336–347 (2004).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Weber, J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol. Immunother. 58, 823–830 (2009).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nature Med. 10, 909–915 (2004).
Beer, T. M. et al. Phase I trial of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. Abstr 26 (Suppl. 15), 5004 (2008).
Ishida, Y., Agata, Y., Shibahara, K. & Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895 (1992).
Dong, H., Zhu, G., Tamada, K. & Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature Med. 5, 1365–1369 (1999).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Hirano, F. et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 65, 1089–1096 (2005).
Iwai, Y., Terawaki, S. & Honjo, T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. 17, 133–144 (2005). References 92 and 93 are among the first to highlight a potential role for PD1 blockade in cancer immunotherapy.
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001).
Nishimura, H., Nose, M., Hiai, H., Minato, N. & Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999).
Thompson, R. H. et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl Acad. Sci. USA 101, 17174–17179 (2004).
Sfanos, K. S. et al. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate 69, 1694–1703 (2009).
Ahmadzadeh, M. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009). References 97 and 98 show that the CD8+ T cells that infiltrate tumours are likely to express PD1, suggesting that PD1 blockade may have therapeutic relevance.
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010). One of the first clinical trials in which the immune checkpoint molecule PD1 was blocked with a monoclonal antibody; these data provided evidence for clinical activity together with a generally benign side-effect profile.
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 92, 205–216 (2000).
Small, E. J. et al. Placebo-controlled Phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 24, 3089–3094 (2006). The first randomized Phase III trial to show a clinical benefit for antigen-specific immunotherapy in patients with prostate cancer.
Kantoff, P. W. et al. Overall survival analysis of a Phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 1099–1105 (2010).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 14 Jun 2010 (doi:10.1056/NEJMoa1003466). The first randomized Phase III trial to show a clinical benefit for blockade of the immune checkpoint molecule CTLA4 in patients with cancer.
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Gulley, J. L. et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol. Immunother. 59, 663–674 (2009). A retrospective analysis showing that virus-based cancer vaccines may be efficacious only in the setting of a low disease burden.
Finn, O. J. Cancer vaccines: between the idea and the reality. Nature Rev. Immunol. 3, 630–641 (2003).
Dudley, M. E. et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 23, 2346–2357 (2005).
Korman, A. J., Peggs, K. S. & Allison, J. P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 90, 297–339 (2006).
Halabi, S. et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J. Clin. Oncol. 21, 1232–1237 (2003).
Hales, R. K. et al. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann. Oncol. 17 Mar 2010 (doi:10.1093/annonc/mdq048).
Ratain, M. J. Phase II oncology trials: let's be positive. Clin. Cancer Res. 11, 5661–5662 (2005).
Higano, C. S. et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 113, 975–984 (2008).
Prell, R. A., Gearin, L., Simmons, A., Vanroey, M. & Jooss, K. The anti-tumor efficacy of a GM-CSF-secreting tumor cell vaccine is not inhibited by docetaxel administration. Cancer Immunol. Immunother. 55, 1285–1293 (2006).
Machiels, J. P. et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 61, 3689–3697 (2001).
Zitvogel, L. et al. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 118, 1991–2001 (2008).
Nesslinger, N. J. et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin. Cancer Res. 13, 1493–1502 (2007).
Morse, M. D. & McNeel, D. G. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum. Immunol. 71, 496–504 (2010).
Sanda, M. G. et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology 53, 260–266 (1999).
Madan, R. A. et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin. Cancer Res. 14, 4526–4531 (2008). These clinical data support the concept that it is important to consider the relative sequencing of conventional treatment (in this case, androgen ablation) with respect to vaccination.
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 13, 1050–1059 (2007). An interesting mechansistic study, showing that certain cancer treatments may prime an immune response through TLR4 agonists released by dying tumour cells.
Lugade, A. A. et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174, 7516–7523 (2005).
Chakraborty, M. et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J. Immunol. 170, 6338–6347 (2003).
Demaria, S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Chakraborty, M. et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 64, 4328–4337 (2004).
Gulley, J. L. et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin. Cancer Res. 11, 3353–3362 (2005).
Harris, T. J. et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate 68, 1319–1329 (2008).
Gerritsen, W. et al. A dose-escalation trial of GM-CSF-gene transduced allogeneic prostate cancer cellular immunotherapy in combination with a fully human anti-CTLA4 antibody (MDX-010, ipilimumab) in patients with metastatic hormone-refractory prostate cancer (MHRPC). Ann. Oncol. 18, 24 (2007).
Blansfield, J. A. et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J. Immunother. 28, 593–598 (2005).
Wolpoe, M. E. et al. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in Her-2/neu-transgenic mice. J. Immunol. 171, 2161–2169 (2003).
Smith, B. D. et al. K562/GM-CSF immunotherapy reduces tumor burden in chronic myeloid leukemia patients with residual disease on imatinib mesylate. Clin. Cancer Res. 16, 338–347 (2010).
Schroder, F. H. et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 360, 1320–1328 (2009).
Andriole, G. L. et al. Mortality results from a randomized prostate-cancer screening trial. N. Engl. J. Med. 360, 1310–1319 (2009).
Greene, K. L. et al. Prostate specific antigen best practice statement: 2009 update. J. Urol. 182, 2232–2241 (2009).
Higano, C. S. et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 113, 975–984 (2008).
Small, E. J. et al. A Phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel vs. docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC). [online]. American Society for Clinical Oncology 2009 Genitourinary Cancers Symposium (2009).
Antonarakis, E. S. & Drake, C. G. Current status of immunological therapies for prostate cancer. Curr. Opin. Urol. 20, 241–246 (2010).
Kipp, R. T. & McNeel, D. G. Immunotherapy for prostate cancer — recent progress in clinical trials. Clin. Adv. Hematol. Oncol. 5, 465–469 (2007).
Mohebtash, M., Gulley, J. L., Madan, R. A., Ferrara, T. & Arlen, P. M. Cancer vaccines: current directions and perspectives in prostate cancer. Curr. Opin. Mol. Ther. 11, 31–36 (2009).
Fong, L. & Small, E. J. Immunotherapy for prostate cancer. Curr. Oncol. Rep. 9, 226–233 (2007).
Slovin, S. F. Pitfalls or promise in prostate cancer immunotherapy — which is winning? Cancer J. 14, 26–34 (2008).
Kantoff, P. et al. Updated survival results of the IMPACT trial of sipuleucel-T for metastatic castration-resistant prostate cancer (CRPC) [Online]. American Society of Clinical Oncology 2010 Genitourinary Cancers Symposium (2010).
Acknowledgements
C.G.D. is a Damon Runyon-Lilly Clinical Investigator. This work was also supported by US National institutes of Health grants R01 CA127153 and P50 CA058236, the Patrick C. Walsh Fund, the David Koch Foundation and the Prostate Cancer Foundation. The author would like to acknowledge D. Pardoll and E. Antonarakis for review of this manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
G.G.D. is a consultant for Bristol-Myers Squibb and Medarex. He has received honoraria from Dendreon Inc. He has intellectual property and/or patents with Medarex and Amplimmune and has stock ownership with Amplimmune.
Related links
Glossary
- Localized disease
-
In prostate cancer, this usually refers to disease that does not extend beyond the prostate gland itself, which can be treated with radiotherapy, surgery or the removal of androgens.
- Recurrent disease
-
Cancer that has returned following primary therapy. Recurrent prostate cancer can be detected by a rising level of prostate-specific antigen only (biochemical recurrence) or by computerised tomography or bone scans (metastatic disease).
- Androgen
-
A type of steroid hormone that controls the male characteristics of vertebrate animals. Testosterone is the best example of an androgen important in prostate cancer, but its metabolite, dihydrotestosterone, is the more potent form in most tissues.
- Chemical castration
-
A therapy to decrease circulating androgen levels through pharmacological intervention. In patients with prostate cancer, this is carried out using leuteinizing hormone-releasing hormone (LHRH) antagonists, which act on the hypothalamus to centrally mediate a decrease in testosterone secretion.
- Surgical castration
-
The removal of the testicles to decrease circulating androgen levels. It should be noted that androgens are also secreted by the adrenal cortex, so that surgical castration does not completely eliminate androgens from the blood.
- Castration-resistant disease
-
Prostate cancer that can be shown to be progressing through a rising prostate-specific antigen level or by imaging studies, despite a low or undetectable level of testosterone in the blood following chemical or surgical castration.
- Surrogate endpoint
-
A biological marker used in a clinical trial to substitute for a clinically relevant endpoint. Some examples include cholesterol level, which can be a surrogate endpoint for studies aiming to reduce the risk of heart disease, or CD4+ T cell count, which can be a surrogate endpoint for reducing the chance of death from opportunistic infections in patients with HIV.
- Autochthonous
-
Arising spontaneously over time. Mouse tumour models that are autochronous may more accurately model the natural immune response to cancers, as evolving cancers are recognized by the immune system and induce a tolerogenic state.
- TRAMP model
-
(transgenic adenocarcinoma of the mouse prostate model). A mouse model of prostate cancer in which prostate cancers arise spontaneously because the SV40 large T antigen is expressed in a prostate-restricted manner, downregulating the tumour suppressor molecules P53 and RB locally.
- Central deletion
-
Self tolerance that is created at the level of the central lymphoid organs. Developing T cells in the thymus, and B cells in the bone marrow, that strongly recognize self antigen face deletion or marked suppression.
- Passive immunotherapy
-
The induction of immunity by the transfer of immunoglobulins or T cells.
- Active immunization
-
The induction of immunity by activation or expansion of the endogenous immune repertoire.
- Primary endpoint
-
The main result that is measured at the end of a clinical trial to determine whether the hypothesis under study has been fulfilled.
- RECIST
-
(Response evaluation criteria in solid tumours). A set of formally defined rules used to measure objective clinical responses in cancer patients treated with a particular therapy. These parameters measure a series of index lesions and quantify whether the lesions have decreased in size.
- Single-armed trial
-
A clinical trial without a concurrent control group.
- Halabi nomogram
-
A model that uses historical data to estimate the survival of patients with progressive prostate cancer after castration.
- T cell receptor excision circles
-
DNA episomes that are normally produced during the thymic maturation of T cells, specifically during recombination of the T cell receptor genes.
- Hypophisitis
-
Inflammation of the pituitary gland, which can be induced in patients with cancer by CTLA4-blocking antibodies. Clinically, hypophysitis is characterized by decreased levels of thyroid hormone, cortisol and other hormones.
Rights and permissions
About this article
Cite this article
Drake, C. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol 10, 580–593 (2010). https://doi.org/10.1038/nri2817
Issue Date:
DOI: https://doi.org/10.1038/nri2817
This article is cited by
-
Association of WHSC1/NSD2 and T-cell infiltration with prostate cancer metastasis and prognosis
Scientific Reports (2023)
-
B7-H3 specific CAR-T cells exhibit potent activity against prostate cancer
Cell Death Discovery (2023)
-
Novel Predicted B-Cell Epitopes of PSMA for Development of Prostate Cancer Vaccine
International Journal of Peptide Research and Therapeutics (2020)
-
CAR-T cell therapy: a potential new strategy against prostate cancer
Journal for ImmunoTherapy of Cancer (2019)
-
Potentiating prostate cancer immunotherapy with oncolytic viruses
Nature Reviews Urology (2018)