Key Points
-
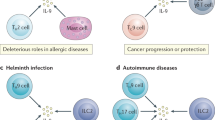
Cross-presentation allows dendritic cells (DCs) to activate CD8+ cytotoxic T lymphocytes (CTLs) for immune defence against viruses that do not infect DCs and tumours that originate from non-DCs.
-
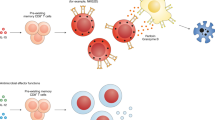
Immunogenic cross-presentation (cross-priming) requires that DCs are licensed by T helper (TH) cells or natural killer T (NKT) cells, which renders them competent to programme CTLs for survival, effector function and memory cell generation.
-
Licensing TH or NKT cells cause DCs to produce discrete chemokines that recruit naive CTLs into lymphatic tissues for cross-priming. Also, DC–TH cell interaction in non-lymphatic tissues results in chemokine production that recruits cross-primed CTLs for cytotoxic effector functions, which promote antiviral defence and immune-mediated disease.
-
Cross-priming allows for the expansion of CTLs and the induction of antiviral immunity even in the presence of viral immune escape from MHC class I presentation in infected cells
-
Cross-presentation of autoantigens causes deletion of autoreactive CTLs that have escaped central tolerance; failure of such cross-tolerance is thought to contribute to the pathogenesis of autoimmune diseases, such as type 1 diabetes, multiple sclerosis and psoriasis.
-
Many tumour antigens are effectively cross-presented but this rarely results in effective cross-priming. Vaccination strategies that combine tumour antigens with adjuvants that mature DCs and increase cross-presentation and chemokine production may overcome this problem when combined with suitable chemotherapies that do not compromise antitumour immunity.
Abstract
Cross-priming is an important mechanism to activate cytotoxic T lymphocytes (CTLs) for immune defence against viruses and tumours. Although it was discovered more than 25 years ago, we have only recently gained insight into the underlying cellular and molecular mechanisms, and we are just beginning to understand its physiological importance in health and disease. Here we summarize current concepts on the cross-talk between the immune cells involved in CTL cross-priming and on its role in antimicrobial and antitumour defence, as well as in immune-mediated diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bevan, M. J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143, 1283–1288 (1976). This is a seminal paper describing the phenomenon of cross-priming.
Huang, A. Y. et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 264, 961–965 (1994).
Sigal, L. J., Crotty, S., Andino, R. & Rock, K. L. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature 398, 77–80 (1999).
Yewdell, J. W. & Haeryfar, S. M. Understanding presentation of viral antigens to CD8+ T cells in vivo: the key to rational vaccine design. Annu. Rev. Immunol. 23, 651–682 (2005).
Kurts, C., Kosaka, H., Carbone, F. R., Miller, J. F. & Heath, W. R. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+T cells. J. Exp. Med. 186, 239–245 (1997). This paper showed the phenomenon of cross-tolerance in a transgenic model, in which cross-presentation of autoantigen causes peripheral deletion of autoreactive CTLs.
Shortman, K. & Naik, S. H. Steady-state and inflammatory dendritic-cell development. Nature Rev. Immunol. 7, 19–30 (2007).
Heath, W. R. & Carbone, F. R. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nature Immunol. 10, 1237–1244 (2009).
Kurts, C., Cannarile, M., Klebba, I. & Brocker, T. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J. Immunol. 166, 1439–1442 (2001).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
del Rio, M. L., Rodriguez-Barbosa, J. I., Kremmer, E. & Forster, R. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 178, 6861–6866 (2007).
Bedoui, S. et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nature Immunol. 10, 488–495 (2009).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 (2010).
den Haan, J. M. & Bevan, M. J. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8+ and CD8− dendritic cells in vivo. J. Exp. Med. 196, 817–827 (2002).
Chung, Y., Chang, J. H., Kweon, M. N., Rennert, P. D. & Kang, C. Y. CD8α−11b+ dendritic cells but not CD8α+ dendritic cells mediate cross-tolerance toward intestinal antigens. Blood 106, 201–206 (2005).
Lutz, M. B. & Kurts, C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur. J. Immunol. 39, 2325–2330 (2009).
Ballesteros-Tato, A., Leon, B., Lund, F. E. & Randall, T. D. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8+ T cell responses to influenza. Nature Immunol. 11, 216–224 (2010).
McDonnell, A. M., Prosser, A. C., van Bruggen, I., Robinson, B. W. & Currie, A. J. CD8α+ DC are not the sole subset cross-presenting cell-associated tumor antigens from a solid tumor. Eur. J. Immunol. 1 Apr 2010 (doi:10.1002/eji.200940153).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Saveanu, L. et al. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science 325, 213–217 (2009).
Burgdorf, S., Kautz, A., Bohnert, V., Knolle, P. A. & Kurts, C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 (2007).
Burgdorf, S. & Kurts, C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol. 20, 89–95 (2008).
Caminschi, I. et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood 112, 3264–3273 (2008).
Weck, M. M. et al. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood 111, 4264–4272 (2008).
Medema, J. P. et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J. Exp. Med. 194, 657–667 (2001).
Bocharov, G., Ford, N. J. & Ludewig, B. A mathematical approach for optimizing dendritic cell-based immunotherapy. Methods Mol. Med. 109, 19–34 (2005).
van Stipdonk, M. J., Lemmens, E. E. & Schoenberger, S. P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nature Immunol. 2, 423–429 (2001).
Kaech, S. M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nature Immunol. 2, 415–422 (2001).
Allan, R. S. et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162 (2006).
Backer, R. et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc. Natl Acad. Sci. USA 107, 216–221 (2010).
Carbone, F. R., Kurts, C., Bennett, S. R., Miller, J. F. & Heath, W. R. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol. Today 19, 368–373 (1998).
Heit, A. et al. CpG-DNA aided cross-priming by cross-presenting B cells. J. Immunol. 172, 1501–1507 (2004).
Bennett, S. R., Carbone, F. R., Toy, T., Miller, J. F. & Heath, W. R. B cells directly tolerize CD8+ T cells. J. Exp. Med. 188, 1977–1983 (1998).
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nature Med. 6, 1348–1354 (2000).
Steinman, R. M. & Pope, M. Exploiting dendritic cells to improve vaccine efficacy. J. Clin. Invest. 109, 1519–1526 (2002).
Maurer, T. et al. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. Eur. J. Immunol. 32, 2356–2364 (2002).
Schulz, O. et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892 (2005).
Burgdorf, S., Scholz, C., Kautz, A., Tampe, R. & Kurts, C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nature Immunol. 9, 558–566 (2008).
van Kooyk, Y. & Rabinovich, G. A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nature Immunol. 9, 593–601 (2008).
Rogers, N. C. et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 (2005).
Barchet, W., Wimmenauer, V., Schlee, M. & Hartmann, G. Accessing the therapeutic potential of immunostimulatory nucleic acids. Curr. Opin. Immunol. 20, 389–395 (2008).
Latz, E. The inflammasomes: mechanisms of activation and function. Curr. Opin. Immunol. 22, 28–23 (2010).
Shi, Y., Evans, J. E. & Rock, K. L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521 (2003).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Hamilton-Williams, E. E. et al. Cutting edge: TLR ligands are not sufficient to break cross-tolerance to self-antigens. J. Immunol. 174, 1159–1163 (2005).
Qiu, F. & Cui, Z. CD4+ T helper cell response is required for memory in CD8+ T lymphocytes induced by a poly(I:C)-adjuvanted MHC I-restricted peptide epitope. J. Immunother. 30, 180–189 (2007).
Melief, C. J. Mini-review: Regulation of cytotoxic T lymphocyte responses by dendritic cells: peaceful coexistence of cross-priming and direct priming? Eur. J. Immunol. 33, 2645–2654 (2003).
Janssen, E. M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003).
Redmond, W. L. & Sherman, L. A. Peripheral tolerance of CD8 T lymphocytes. Immunity 22, 275–284 (2005).
Heymann, F. et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J. Clin. Invest. 119, 1286–1297 (2009). This report shows that cross-talk between DCs and autoreactive T H cells in non-lymphoid tissues causes chemokine production and recruitment of cross-primed autoreactive CTLs that cause immunopathology.
Smith, C. M. et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nature Immunol. 5, 1143–1148 (2004). This paper formally showed that T cells can license DCs for cross-priming of CTLs.
van Mierlo, G. J. et al. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J. Immunol. 173, 6753–6759 (2004).
Sun, J. C., Williams, M. A. & Bevan, M. J. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nature Immunol. 5, 927–933 (2004).
Zehn, D., Lee, S. Y. & Bevan, M. J. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214 (2009).
Belz, G. T., Bedoui, S., Kupresanin, F., Carbone, F. R. & Heath, W. R. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nature Immunol. 8, 1060–1066 (2007).
Keller, A. M., Xiao, Y., Peperzak, V., Naik, S. H. & Borst, J. Costimulatory ligand CD70 allows induction of CD8+ T-cell immunity by immature dendritic cells in a vaccination setting. Blood 113, 5167–5175 (2009).
Keir, M. E., Freeman, G. J. & Sharpe, A. H. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 179, 5064–5070 (2007).
Mescher, M. F. et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 211, 81–92 (2006).
Williams, M. A., Tyznik, A. J. & Bevan, M. J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441, 890–893 (2006).
Spierings, D. C., Lemmens, E. E., Grewal, K., Schoenberger, S. P. & Green, D. R. Duration of CTL activation regulates IL-2 production required for autonomous clonal expansion. Eur. J. Immunol. 36, 1707–1717 (2006).
Mintern, J. D., Davey, G. M., Belz, G. T., Carbone, F. R. & Heath, W. R. Cutting edge: precursor frequency affects the helper dependence of cytotoxic T cells. J. Immunol. 168, 977–980 (2002).
Fuse, S. et al. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J. Immunol. 182, 4244–4254 (2009).
Peperzak, V., Xiao, Y., Veraar, E. A. & Borst, J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J. Clin. Invest. 120, 168–178 (2009).
Janssen, E. M. et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 (2005).
Sacks, J. A. & Bevan, M. J. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J. Immunol. 180, 4570–4576 (2008).
Castellino, F. et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440, 890–895 (2006). This paper showed that T H cell-mediated licensing of DCs causes CCR5 ligand-mediated recruitment of naive CTLs for cross-priming.
Hugues, S. et al. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nature Immunol. 8, 921–930 (2007).
Dorner, B. G. et al. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity 31, 823–833 (2009).
Fujii, S., Shimizu, K., Hemmi, H. & Steinman, R. M. Innate Vα14+ natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol. Rev. 220, 183–198 (2007).
Semmling, V. et al. Alternative cross-priming through CCL17–CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nature Immunol. 11, 313–320 (2010). This paper showed that NKT cells can license DCs for cross-priming and recruit naive CTLs by CCR4 ligands.
Wakim, L. M., Waithman, J., van Rooijen, N., Heath, W. R. & Carbone, F. R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
Nakanishi, Y., Lu, B., Gerard, C. & Iwasaki, A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature 462, 510–513 (2009). This report showed that cross-talk between DCs and T H cells in viral infections of mucosal tissue causes chemokine-mediated tissue recruitment of cross-primed CTLs.
Marzo, A. L. et al. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J. Immunol. 165, 6047–6055 (2000).
Savinov, A. Y., Wong, F. S., Stonebraker, A. C. & Chervonsky, A. V. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J. Exp. Med. 197, 643–656 (2003).
Galea, I. et al. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J. Exp. Med. 204, 2023–2030 (2007).
von Oppen, N. et al. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology 49, 1664–1672 (2009).
Dong, H. et al. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity 20, 327–336 (2004).
Reddehase, M. J. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nature Rev. Immunol. 2, 831–844 (2002).
Andrews, D. M., Andoniou, C. E., Granucci, F., Ricciardi-Castagnoli, P. & Degli-Esposti, M. A. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nature Immunol. 2, 1077–1084 (2001).
Holtappels, R. et al. Cytomegalovirus misleads its host by priming of CD8 T cells specific for an epitope not presented in infected tissues. J. Exp. Med. 199, 131–136 (2004).
Bickham, K. et al. Dendritic cells initiate immune control of Epstein-Barr virus transformation of B lymphocytes in vitro. J. Exp. Med. 198, 1653–1663 (2003).
Bohm, V. et al. Epitope-specific in vivo protection against cytomegalovirus disease by CD8 T cells in the murine model of preemptive immunotherapy. Med. Microbiol. Immunol. 197, 135–144 (2008).
Tewalt, E. F. et al. Viral sequestration of antigen subverts cross presentation to CD8+ T cells. PLoS Pathog. 5, e1000457 (2009).
Le Bon, A. et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nature Immunol. 4, 1009–1015 (2003). This paper revealed that type I IFN stimulates cross-priming.
Lang, K. S. et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J. Clin. Invest. 116, 2456–2463 (2006).
Rehermann, B. & Nascimbeni, M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature Rev. Immunol. 5, 215–229 (2005).
Hosel, M. et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology 50, 1773–1782 (2009).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Datta, S. K. et al. Vaccination with irradiated Listeria induces protective T cell immunity. Immunity 25, 143–152 (2006).
Neuenhahn, M. et al. CD8α+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity 25, 619–630 (2006).
Kang, S. J., Liang, H. E., Reizis, B. & Locksley, R. M. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity 29, 819–833 (2008).
Tseng, K. E., Chung, C. Y., H'Ng, W., S. & Wang, S. L. Early infection termination affects number of CD8+ memory T cells and protective capacities in Listeria monocytogenes-infected mice upon rechallenge. J. Immunol. 182, 4590–4600 (2009).
Schaible, U. E. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nature Med. 9, 1039–1046 (2003).
Winau, F. et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 24, 105–117 (2006).
Zehn, D. & Bevan, M. J. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity 25, 261–270 (2006).
McDevitt, H. O. & Unanue, E. R. Autoimmune diabetes mellitus — much progress, but many challenges. Adv. Immunol. 100, 1–12 (2008).
de Jersey, J. et al. β cells cannot directly prime diabetogenic CD8 T cells in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 104, 1295–1300 (2007). This paper showed that cross-priming is required for diabetes in the NOD mouse model.
Hamilton-Williams, E. E. et al. Expression of diabetes-associated genes by dendritic cells and CD4 T cells drives the loss of tolerance in nonobese diabetic mice. J. Immunol. 183, 1533–1541 (2009).
von Herrath, M. Diabetes: A virus-gene collaboration. Nature 459, 518–519 (2009).
Richer, M. J. & Horwitz, M. S. Coxsackievirus infection as an environmental factor in the etiology of type 1 diabetes. Autoimmun Rev. 8, 611–615 (2009).
Benoist, C. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nature Immunol. 2, 797–801 (2001).
Pinkse, G. G. et al. Autoreactive CD8 T cells associated with β cell destruction in type 1 diabetes. Proc. Natl Acad. Sci. USA 102, 18425–18430 (2005).
Nakayama, M. et al. Priming and effector dependence on insulin B: 9–23 peptide in NOD islet autoimmunity. J. Clin. Invest. 117, 1835–1843 (2007).
Luckashenak, N. et al. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity 28, 521–532 (2008). This report showed that non-transgenic self antigens can induce cross-tolerance.
Hoglund, P. et al. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J. Exp. Med. 189, 331–339 (1999).
Mohan, J. F. et al. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nature Immunol. 4, 350–354 (2010).
Jahromi, M. M. & Eisenbarth, G. S. Cellular and molecular pathogenesis of type 1A diabetes. Cell. Mol. Life Sci. 64, 865–72 (2007).
Neilson, E. G., McCafferty, E., Mann, R., Michaud, L. & Clayman, M. Murine interstitial nephritis. III. The selection of phenotypic (Lyt and L3T4) and idiotypic (RE-Id) T cell preferences by genes in Igh-1 and H-XXXXX2K characterizes the cell-mediated potential for disease expression: susceptible mice provide a unique effector T cell repertoire in response to tubular antigen. J. Immunol. 134, 2375–2382 (1985).
Gudjonsson, J. E., Johnston, A., Sigmundsdottir, H. & Valdimarsson, H. Immunopathogenic mechanisms in psoriasis. Clin. Exp. Immunol. 135, 1–8 (2004).
Goverman, J. Autoimmune T cell responses in the central nervous system. Nature Rev. Immunol. 9, 393–407 (2009).
Perchellet, A., Brabb, T. & Goverman, J. M. Crosspresentation by nonhematopoietic and direct presentation by hematopoietic cells induce central tolerance to myelin basic protein. Proc. Natl Acad. Sci. USA 105, 14040–14045 (2008). This paper implicated cross-tolerance in the control of encephalitogenic CTLs.
Huseby, E. S. et al. A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J. Exp. Med. 194, 669–676 (2001).
Harkiolaki, M. et al. T cell-mediated autoimmune disease due to low-affinity crossreactivity to common microbial peptides. Immunity 30, 348–357 (2009).
Fiorillo, M. T. & Sorrentino, R. T-cell responses against viral and self-epitopes and HLA-B27 subtypes differentially associated with ankylosing spondylitis. Adv. Exp. Med. Biol. 649, 255–262 (2009).
Ichiki, Y. et al. T cell immunity in autoimmune hepatitis. Autoimmun. Rev. 4, 315–321 (2005).
Kammer, A. R. et al. Molecular mimicry of human cytochrome P450 by hepatitis C virus at the level of cytotoxic T cell recognition. J. Exp. Med. 190, 169–176 (1999).
Gokmen, M. R., Lombardi, G. & Lechler, R. I. The importance of the indirect pathway of allorecognition in clinical transplantation. Curr. Opin. Immunol. 20, 568–574 (2008).
Boon, T., Cerottini, J. C., Van den Eynde, B., van der Bruggen, P. & Van Pel, A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 12, 337–365 (1994).
Boland, C. R. & Ricciardiello, L. How many mutations does it take to make a tumor? Proc. Natl Acad. Sci. USA 96, 14675–14677 (1999).
Marzo, A. L. et al. Tumor antigens are constitutively presented in the draining lymph nodes. J. Immunol. 162, 5838–5845 (1999).
Cuenca, A. et al. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res. 63, 9007–9015 (2003).
Ney, J. T. et al. Autochthonous liver tumors induce systemic T cell tolerance associated with T cell receptor down-modulation. Hepatology 49, 471–481 (2009).
Gerner, M. Y., Casey, K. A. & Mescher, M. F. Defective MHC class II presentation by dendritic cells limits CD4 T cell help for antitumor CD8 T cell responses. J. Immunol. 181, 155–164 (2008).
Stumbles, P. A. et al. Cutting edge: tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J. Immunol. 173, 5923–5928 (2004).
Nelson, D. J. et al. Tumor progression despite efficient tumor antigen cross-presentation and effective “arming” of tumor antigen-specific CTL. J. Immunol. 166, 5557–5566 (2001).
Lyman, M. A., Aung, S., Biggs, J. A. & Sherman, L. A. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J. Immunol. 172, 6558–6567 (2004).
Nguyen, L. T. et al. Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance. J. Exp. Med. 195, 423–435 (2002).
Nowak, A. K. et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol. 170, 4905–4913 (2003).
Sauter, B. et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423–434 (2000).
Schnurr, M. et al. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and γδ T cells. Cancer Res. 62, 2347–2352 (2002).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Med. 13, 54–61 (2007).
Lake, R. A. & Robinson, B. W. Immunotherapy and chemotherapy — a practical partnership. Nature Rev. Cancer 5, 397–405 (2005).
Schnurr, M. et al. ISCOMATRIX adjuvant induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase II. J. Immunol. 182, 1253–1259 (2009).
Motta, I. et al. Cross-presentation by dendritic cells of tumor antigen expressed in apoptotic recombinant canarypox virus-infected dendritic cells. J. Immunol. 167, 1795–1802 (2001).
Bendz, H. et al. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J. Biol. Chem. 282, 31688–31702 (2007).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 13, 1050–1059 (2007).
Dhodapkar, K. M., Krasovsky, J., Williamson, B. & Dhodapkar, M. V. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J. Exp. Med. 195, 125–133 (2002).
Zhang, H. et al. Comparing pooled peptides with intact protein for accessing cross-presentation pathways for protective CD8+ and CD4+ T cells. J. Biol. Chem. 284, 9184–9191 (2009).
Melief, C. J. & van der Burg, S. H. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nature Rev. Cancer 8, 351–360 (2008).
Kenter, G. G. et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 361, 1838–1847 (2009).
Bretscher, P. & Cohn, M. A theory of self–nonself discrimination. Science 169, 1042–1049 (1970).
Bousso, P. & Albert, M. L. Signal 0 for guided priming of CTLs: NKT cells do it too. Nature Immunol. 11, 284–286 (2010).
Robson, N. C., Hoves, S., Maraskovsky, E. & Schnurr, M. Presentation of tumour antigens by dendritic cells and challenges faced. Curr. Opin. Immunol. 22, 137–144 (2010).
Guermonprez, P. et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003).
Houde, M. et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402–406 (2003).
Touret, N. et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell 123, 157–170 (2005).
Savina, A. et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126, 205–218 (2006).
Di Pucchio, T. et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nature Immunol. 9, 551–557 (2008).
Belizaire, R. & Unanue, E. R. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc. Natl Acad. Sci. USA 106, 17463–17468 (2009).
Zinkernagel, R. M. On cross-priming of MHC class I-specific CTL: rule or exception? Eur. J. Immunol. 32, 2385–2392 (2002).
Wolkers, M. C., Brouwenstijn, N., Bakker, A. H., Toebes, M. & Schumacher, T. N. Antigen bias in T cell cross-priming. Science 304, 1314–1317 (2004).
Burgdorf, S. et al. Steady-state cross-presentation of OVA is mannose receptor-dependent but inhibitable by collagen fragments. Proc. Natl Acad. Sci. USA 107, E48–E49 (2010).
Di Bonito, P. et al. Anti-tumor CD8+ T cell immunity elicited by HIV-1-based virus-like particles incorporating HPV-16 E7 protein. Virology 395, 45–55 (2009).
Kemball, C. C. et al. Coxsackievirus B3 inhibits antigen presentation in vivo, exerting a profound and selective effect on the MHC class I pathway. PLoS Pathog. 5, e1000618 (2009).
Racanelli, V., Behrens, S. E., Aliberti, J. & Rehermann, B. Dendritic cells transfected with cytopathic self-replicating RNA induce crosspriming of CD8+ T cells and antiviral immunity. Immunity 20, 47–58 (2004).
Ramirez, M. C. & Sigal, L. J. Macrophages and dendritic cells use the cytosolic pathway to rapidly cross-present antigen from live, vaccinia-infected cells. J. Immunol. 169, 6733–6742 (2002).
Lundie, R. J. et al. Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8α+ dendritic cells. Proc. Natl Acad. Sci. USA 105, 14509–14514 (2008).
Araujo, A. F. et al. CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2. Infect. Immun. 73, 6017–6025 (2005).
Steele, L. N., Balsara, Z. R. & Starnbach, M. N. Hematopoietic cells are required to initiate a Chlamydia trachomatis-specific CD8+ T cell response. J. Immunol. 173, 6327–6337 (2004).
Calzascia, T. et al. Cutting edge: cross-presentation as a mechanism for efficient recruitment of tumor-specific CTL to the brain. J. Immunol. 171, 2187–2191 (2003).
Brazillet, M. P., Batteux, F., Abehsira-Amar, O., Nicoletti, F. & Charreire, J. Induction of experimental autoimmune thyroiditis by heat-denatured porcine thyroglobulin: a Tc1-mediated disease. Eur. J. Immunol. 29, 1342–1352 (1999).
Sotomayor, E. M. et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood 98, 1070–1077 (2001).
Li, Y. et al. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 68, 6889–6895 (2008).
Acknowledgements
The authors thank W. Kolanus (LIMES Institute Bonn), J. Yewdell (NIH), M. von Herrath (LIAI) and R. Slattery (Monash University Melbourne) for comments and discussions. We apologize to all colleagues whose work could not be cited owing to space restrictions. The authors are supported by the German Research foundation (DFG Sonderforschungsbereiche 704, 670 and 645, Transregio 57, Klinische Forschergruppe 228), the German Academic Exchange service (DAAD) and the Group of Eight, Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Cross-presentation
-
The ability of certain antigen-presenting cells to load peptides that are derived from exogenous antigens onto MHC class I molecules. This property is atypical, because most cells exclusively present peptides from their endogenous proteins on MHC class I molecules. Cross-presentation can lead to cross-priming or cross-tolerance.
- Cross-priming
-
The initiation of an immunogenic CD8+ T cell response to an antigen that is not synthesized by the antigen-presenting cell. Cross-presentation is essential for the initiation of immune responses to viruses that do not infect antigen-presenting cells.
- Cross-tolerance
-
The antigen-specific tolerization of CD8+ T cells by antigen-presenting cells that cross-present self or innocuous antigens, which results in BIM-mediated (BCL-2 inhibitable) deletion of autoreactive CD8+ T cells.
- C-type lectin receptors
-
A large family of receptors that bind glycosylated ligands and have several roles, such as in cell adhesion, endocytosis, pathogen recognition, natural killer cell target recognition and dendritic cell activation.
- Inflammasome
-
A large multiprotein complex formed by a nucleotide-binding domain (NBD)-, leucine-rich repeat (LRR)-containing family (NLR) protein, the adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and pro-caspase 1. The assembly of the inflammasome leads to the activation of caspase 1, which cleaves pro-IL-1β and pro-IL-18 to generate the active pro-inflammatory cytokines.
- DC licensing
-
A concept that DCs must be converted by an antigen-specific T helper cell into a functional state required for immunogenic activation of CTLs, which decreases the likelihood of autoimmunity, but requires coordination of cell encounters by chemokines.
- CTL programming
-
A concept that CTLs receive additional information during their activation that affects their effector functions, life-span, memory differentiation or migratory properties. These signals determine whether cross-presentation leads to cross-priming or cross-tolerance.
- Central tolerance
-
Self-tolerance that is created at the level of the central lymphoid organs. Developing T cells in the thymus and B cells in the bone marrow that strongly recognize self antigen face deletion or marked suppression.
- Antigenic mimicry
-
A mechanism for the induction of autoimmunity, in which a pathogen expresses a protein or peptide that is similar to a self protein. After the induction of a pathogen-specific immune response, a cross-reactive response to self results in autoimmune pathology.
- Oligodendrocyte
-
A type of glial cell that creates the myelin sheath that insulates axons and improves the speed and reliability of signal transmission by neurons.
- Signals 1 and 2
-
T cells require presentation of antigen (signal 1) and co-stimulatory signals (signal 2) for immunogenic activation; cytokines have been proposed to be a third signal that dictates T cell differentiation.
Rights and permissions
About this article
Cite this article
Kurts, C., Robinson, B. & Knolle, P. Cross-priming in health and disease. Nat Rev Immunol 10, 403–414 (2010). https://doi.org/10.1038/nri2780
Issue Date:
DOI: https://doi.org/10.1038/nri2780
This article is cited by
-
Incorporating the Molecular Mimicry of Environmental Antigens into the Causality of Autoimmune Hepatitis
Digestive Diseases and Sciences (2023)
-
Differential centrifugation enhances the anti-tumor immune effect of tumor lysate-pulsed dendritic cell vaccine against glioblastoma*
Oncology and Translational Medicine (2022)
-
A lysosome-targeted DNA nanodevice selectively targets macrophages to attenuate tumours
Nature Nanotechnology (2021)
-
Kidney dendritic cells: fundamental biology and functional roles in health and disease
Nature Reviews Nephrology (2020)
-
Combined Rho-kinase inhibition and immunogenic cell death triggers and propagates immunity against cancer
Nature Communications (2018)