Key Points
-
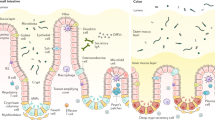
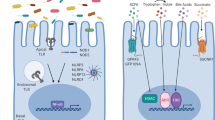
A single layer of intestinal epithelial cells (IECs) provides a physical barrier that separates trillions of commensal bacteria in the intestinal lumen from the underlying lamina propria and deeper intestinal layers. IECs express several Toll-like receptors (TLRs) including TLR1, TLR2, TLR4, TLR5 and TLR9.
-
Polarization of TLRs to the basolateral membrane of the IECs has been described for TLR4, TLR5 and TLR9 and decreases activation in response to luminal commensal bacteria. TLRs are also expressed by specific types of IEC within the crypt to villus axis, such as enteroendocrine cells, which respond to TLR activation by secreting somatostatin and cholecystokinin.
-
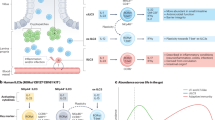
Activation of TLRs in IECs results in proliferation, restitution and protection against apoptosis. Following intestinal injury, TLR4 signalling through myeloid differentiation primary-response protein 88 (MYD88) results in the induction of cyclooxygenase 2 (COX2) expression and the production of prostaglandin E2 (PGE2) and amphiregulin, which is an epidermal growth factor family member.
-
TLRs regulate stem cell proliferation during injury by directing the recruitment of COX2-expressing mesenchymal stromal cells and macrophages, which produce trophic factors including PGE2 that stimulate stem cell proliferation.
-
Paneth cells are specialized epithelial cells located at the base of the small intestinal crypts that produce antimicrobial proteins and lectins. MYD88 signalling by Paneth cells is required for the expression of regenerating islet-derived protein 3γ (REG3γ), REG3β, CRP-ductin and resistin-like molecule-β (RELMβ). The absence of these lectins allows dissemination of intestinal pathogens.
-
Secretory IgA produced by lamina propria B cells protects against invasion of both commensal organisms and pathogens. TLR signalling by IECs leads to the production of cytokines such as a proliferation-inducing ligand (APRIL), which induce class switching to IgA2, a protease-resistant, T cell-independent isotype of IgA.
-
TLR signalling promotes the development of APC-dependent colorectal cancers and inflammation-associated colorectal cancers. Manipulating TLR signalling may have a beneficial effect in this setting.
Abstract
A single layer of epithelial cells lines the small and large intestines and functions as a barrier between commensal bacteria and the rest of the body. Ligation of Toll-like receptors (TLRs) on intestinal epithelial cells by bacterial products promotes epithelial cell proliferation, secretion of IgA into the gut lumen and expression of antimicrobial peptides. As described in this Review, this establishes a microorganism-induced programme of epithelial cell homeostasis and repair in the intestine. Dysregulation of this process can result in chronic inflammatory and over-exuberant repair responses, and it is associated with the development of colon cancer. Thus, dysregulated TLR signalling by intestinal epithelial cells may explain how colonic bacteria and inflammation promote colorectal cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
02 February 2010
In the version of the article initially published, figure 1 depicted Paneth cells at the base of the crypt in the colon. These cells should have been enterocytes. This has been corrected in the HTML and PDF versions of this article.
References
Yen, T. H. & Wright, N. A. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2, 203–212 (2006).
Jabri, B. & Ebert, E. Human CD8+ intraepithelial lymphocytes: a unique model to study the regulation of effector cytotoxic T lymphocytes in tissue. Immunol. Rev. 215, 202–214 (2007).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2, 361–367 (2001).
Niess, J. H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Khan, M. A. et al. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect. Immun. 74, 2522–2536 (2006).
Lebeis, S. L., Bommarius, B., Parkos, C. A., Sherman, M. A. & Kalman, D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 179, 566–577 (2007).
Slack, E. et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 325, 617–620 (2009).
Cario, E. & Podolsky, D. K. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68, 7010–7017 (2000).
Abreu, M. T. et al. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167, 1609–1616 (2001).
Otte, J. M., Cario, E. & Podolsky, D. K. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126, 1054–1070 (2004).
Vamadevan, A. S. et al. Regulation of TLR4-associated MD-2 in intestinal epithelial cells: a comprehensive analysis. Innate Immun. 26 Aug 2009 (doi:10.1177/1753425909339231).
Abreu, M. T. et al. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J. Biol. Chem. 277, 20431–20437 (2002).
Pedersen, G., Andresen, L., Matthiessen, M. W., Rask-Madsen, J. & Brynskov, J. Expression of Toll-like receptor 9 and response to bacterial CpG oligodeoxynucleotides in human intestinal epithelium. Clin. Exp. Immunol. 141, 298–306 (2005).
Rehli, M. et al. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J. Biol. Chem. 275, 9773–9781 (2000).
Suzuki, M., Hisamatsu, T. & Podolsky, D. K. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4–MD-2 complex. Infect. Immun. 71, 3503–3511 (2003).
Mueller, T., Terada, T., Rosenberg, I. M., Shibolet, O. & Podolsky, D. K. Th2 cytokines down-regulate TLR expression and function in human intestinal epithelial cells. J. Immunol. 176, 5805–5814 (2006).
Dalpke, A. & Heeg, K. Signal integration following Toll-like receptor triggering. Crit. Rev. Immunol. 22, 217–250 (2002).
Lotz, M. et al. Cytokine-mediated control of lipopolysaccharide-induced activation of small intestinal epithelial cells. Immunology 122, 306–315 (2007).
Lundin, A. et al. Gut flora, Toll-like receptors and nuclear receptors: a tripartite communication that tunes innate immunity in large intestine. Cell. Microbiol. 10, 1093–1103 (2008). This study shows that in the absence of commensal flora, mice have lower IEC expression of TLRs and are more susceptible to pathogenic infection.
Ewaschuk, J. B. et al. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect. Immun. 75, 2572–2579 (2007).
Moran, G. W., Leslie, F. C., Levison, S. E. & McLaughlin, J. T. Review: enteroendocrine cells: neglected players in gastrointestinal disorders? Therap. Adv. Gastroenterol. 1, 51–60 (2008).
Bogunovic, M. et al. Enteroendocrine cells express functional Toll-like receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1770–G1783 (2007).
Palazzo, M. et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J. Immunol. 178, 4296–4303 (2007).
Chabot, S., Wagner, J. S., Farrant, S. & Neutra, M. R. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J. Immunol. 176, 4275–4283 (2006).
Fusunyan, R. D., Nanthakumar, N. N., Baldeon, M. E. & Walker, W. A. Evidence for an innate immune response in the immature human intestine: Toll-like receptors on fetal enterocytes. Pediatr. Res. 49, 589–593 (2001).
Gewirtz, A. T., Navas, T. A., Lyons, S., Godowski, P. J. & Madara, J. L. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885 (2001).
Rhee, S. H. et al. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc. Natl Acad. Sci. USA 102, 13610–13615 (2005). This study shows that TLR5 is expressed on the basolateral surface in human colon IECs and is therefore only activated when the epithelial barrier has been damaged, which limits responses to commensal organisms.
Lee, J. et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nature Cell Biol. 8, 1327–1336 (2006). This study shows that the intracellular localization of TLRs in IECs differs from that in haematopoietic cells and that basolateral and apical recognition of PAMPs by TLR9 triggers distinct signalling programmes.
Bambou, J.C. et al. In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J. Biol. Chem. 279, 42984–42992 (2004).
Vijay-Kumar, M. et al. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 117, 3909–3921 (2007).
Uematsu, S. et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature Immunol. 9, 769–776 (2008).
Lenoir, C. et al. MD-2 controls bacterial lipopolysaccharide hyporesponsiveness in human intestinal epithelial cells. Life Sci. 82, 519–528 (2008).
Cario, E. et al. Commensal-associated molecular patterns induce selective Toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am. J. Pathol. 160, 165–173 (2002).
Latz, E. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nature Immunol. 5, 190–198 (2004).
Leifer, C. A. et al. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J. Immunol. 173, 1179–1183 (2004).
Akhtar, M., Watson, J. L., Nazli, A. & McKay, D. M. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-κB-independent pathway. FASEB J. 17, 1319–1321 (2003).
Rachmilewitz, D. et al. Immunostimulatory oligonucleotides inhibit colonic proinflammatory cytokine production in ulcerative colitis. Inflamm. Bowel Dis. 12, 339–345 (2006).
Park, B. S. et al. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature 458, 1191–1195 (2009).
Saitoh, S. & Miyake, K. Regulatory molecules required for nucleotide-sensing Toll-like receptors. Immunol. Rev. 227, 32–43 (2009).
Hornef, M. W., Frisan, T., Vandewalle, A., Normark, S. & Richter-Dahlfors, A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J. Exp. Med. 195, 559–570 (2002).
Hornef, M. W., Normark, B. H., Vandewalle, A. & Normark, S. Intracellular recognition of lipopolysaccharide by Toll-like receptor 4 in intestinal epithelial cells. J. Exp. Med. 198, 1225–1235 (2003).
Burns, K. et al. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nature Cell Biol. 2, 346–351 (2000).
Zhang, G. & Ghosh, S. Negative regulation of Toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 277, 7059–7065 (2002).
Melmed, G. et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170, 1406–1415 (2003).
Steenholdt, C., Andresen, L., Pedersen, G., Hansen, A. & Brynskov, J. Expression and function of Toll-like receptor 8 and Tollip in colonic epithelial cells from patients with inflammatory bowel disease. Scand. J. Gastroenterol. 44, 195–204 (2009).
Wald, D. et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nature Immunol. 4, 920–927 (2003).
Garlanda, C. et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc. Natl Acad. Sci. USA 101, 3522–3526 (2004).
Xiao, H. et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity 26, 461–475 (2007).
Dubuquoy, L. et al. Impaired expression of peroxisome proliferator-activated receptor γ in ulcerative colitis. Gastroenterology 124, 1265–1276 (2003).
Shimazu, R. et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189, 1777–1782 (1999).
Cario, E. et al. Trypsin-sensitive modulation of intestinal epithelial MD-2 as mechanism of lipopolysaccharide tolerance. J. Immunol. 176, 4258–4266 (2006).
Scoville, D. H., Sato, T., He, X. C. & Li, L. Current view: intestinal stem cells and signaling. Gastroenterology 134, 849–864 (2008).
Abrams, G. D., Bauer, H. & Sprinz, H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab. Invest. 12, 355–364 (1963).
Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I. & Stappenbeck, T. S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl Acad. Sci. USA 102, 99–104 (2005). This study shows that decreased IEC proliferation in germ-free mice is due to defective TLR signalling by macrophages that secrete trophic factors that lead to stem cell proliferation.
Nowacki, W., Cederblad, B., Renard, C., La Bonnardiere, C. & Charley, B. Age-related increase of porcine natural interferon α producing cell frequency and of interferon yield per cell. Vet. Immunol. Immunopathol. 37, 113–122 (1993).
Fukata, M. et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1055–G1065 (2005).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Okayasu, I. et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98, 694–702 (1990).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004). This paper shows that TLR signalling is involved in repair of the intestinal barrier following injury. Animals deficient in MYD88, TLR4 or TLR2 had higher mortality than wild-type mice following DSS-induced colitis. Treatment of wild-type mice with antibiotics made them susceptible to colitis.
Araki, A. et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J. Gastroenterol. 40, 16–23 (2005).
Abreu, M. T., Fukata, M. & Arditi, M. TLR signaling in the gut in health and disease. J. Immunol. 174, 4453–4460 (2005).
Brown, S. L. et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J. Clin. Invest. 117, 258–269 (2007). This study indicates that following injury of the intestine, proliferation of IECs requires the repositioning of COX2-expressing mesenchymal stromal cells to the stem cell niche. Mesenchymal stromal cell repositioning and PGE 2 production required MYD88, suggesting that TLR signalling is involved in regulating proliferation through the stem cell niche.
Ungaro, R. et al. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1167–G1179 (2009).
Fort, M. M. et al. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J. Immunol. 174, 6416–6423 (2005).
Zheng, L., Riehl, T. & Stenson, W. F. Regulation of colonic epithelial repair in mice by Toll-like receptors and hyaluronic acid. Gastroenterology 6, 2041–2051 (2009).
Fukata, M. et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology 131, 862–877 (2006). This study indicates that TLR4-deficient IECs fail to upregulate COX2 expression following injury. The absence of COX2 and PGE 2 results in decreased IEC proliferation, implicating an autocrine effect of TLR4 signalling in IECs in promoting proliferation in response to injury.
Fukata, M. et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm. Bowel Dis. 15, 997–1006 (2009).
Giraud, A. S. X. Trefoil peptide and EGF receptor/ligand transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G501–G506 (2000).
Fukata, M. et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 133, 1869–1881 (2007). In addition to reference 66, this study shows that TLR4-deficient mice are protected against colitis-associated neoplasia. This has implications for targeting TLR4 in dysplasia and cancer in patients with ulcerative colitis.
Vijay-Kumar, M. et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J. Immunol. 180, 8280–8285 (2008).
Cario, E., Gerken, G. & Podolsky, D. K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132, 1359–1374 (2007).
Vijay-Kumar, M. et al. Activation of Toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm. Bowel Dis. 13, 856–864 (2007).
Rachmilewitz, D. et al. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 122, 1428–1441 (2002).
Rachmilewitz, D. et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126, 520–528 (2004).
Katakura, K. et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Invest. 115, 695–702 (2005). This study shows that TLR9 signalling in the intestine results in the production of IFNα or IFNβ, not TNF as occurs in macrophages, and protects against colitis.
Vijay-Kumar, M. et al. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am. J. Pathol. 169, 1686–1700 (2006).
Yu, Y. et al. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagellin-induced proinflammatory gene expression. J. Immunol. 176, 6194–6201 (2006).
Zeng, H. et al. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G96–G108 (2006).
Mohiuddin, M. et al. Preoperative chemoradiation in fixed distal rectal cancer: dose time factors for pathological complete response. Int. J. Radiat. Oncol. Biol. Phys. 46, 883–888 (2000).
Lee, C. M., Lee, R. J., Handrahan, D. L. & Sause, W. T. Comparison of late rectal toxicity from conventional versus three-dimensional conformal radiotherapy for prostate cancer: analysis of clinical and dosimetric factors. Urology 65, 114–119 (2005).
Manichanh, C. et al. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am. J. Gastroenterol. 103, 1754–1761 (2008).
Riehl, T., Cohn, S., Tessner, T., Schloemann, S. & Stenson, W. F. Lipopolysaccharide is radioprotective in the mouse intestine through a prostaglandin-mediated mechanism. Gastroenterology 118, 1106–1116 (2000).
Riehl, T. E., Newberry, R. D., Lorenz, R. G. & Stenson, W. F. TNFR1 mediates the radioprotective effects of lipopolysaccharide in the mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G166–G173 (2004).
Burdelya, L. G. et al. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 320, 226–230 (2008). This study shows that administration of synthetic flagellin protects the intestine from radiation-induced apoptosis, identifying a potential clinical application for TLR ligands.
Sanders, C. J., Moore, D. A., Williams, I. R. & Gewirtz, A. T. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J. Immunol. 180, 7184–7192 (2008).
Persing, D. H. et al. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10, S32–S37 (2002).
Soraisham, A. S., Amin, H. J., Al-Hindi, M. Y., Singhal, N. & Sauve, R. S. Does necrotising enterocolitis impact the neurodevelopmental and growth outcomes in preterm infants with birthweight < or =1250 g? J. Paediatr. Child Health 42, 499–504 (2006).
Guner, Y. S. et al. Necrotizing enterocolitis — bench to bedside: novel and emerging strategies. Semin. Pediatr. Surg. 17, 255–265 (2008).
Jilling, T. et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 177, 3273–3282 (2006).
Leaphart, C. L. et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 (2007).
Lotz, M. et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 203, 973–984 (2006). This study shows that following colonization with maternal flora, neonatal mice have decreased IEC responsiveness to LPS, due in part to post-transcriptional downregulation of IRAK1. This observation may explain why premature infants develop NEC, in which TLR4 signalling seems to have a detrimental role.
Liu, Y. et al. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G442–G450 (2009).
Sodhi, C. P. et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired β-catenin signaling in necrotizing enterocolitis. Gastroenterology 138, 185–196 (2009).
Gribar, S. C. et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J. Immunol. 182, 636–646 (2009).
Le Mandat Schultz, A. et al. Expression of TLR-2, TLR-4, NOD2 and pNF-κB in a neonatal rat model of necrotizing enterocolitis. PLoS ONE 2, e1102 (2007).
Lin, H. C. et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 122, 693–700 (2008).
Hooper, L. V. et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001).
Cario, E., Gerken, G. & Podolsky, D. K. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127, 224–238 (2004).
Gibson, D. L. et al. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10, 388–403 (2008).
Vora, P. et al. β-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J. Immunol. 173, 5398–5405 (2004).
Selsted, M. E. & Ouellette, A. J. Mammalian defensins in the antimicrobial immune response. Nature Immunol. 6, 551–557 (2005).
Hooper, L. V., Stappenbeck, T. S., Hong, C. V. & Gordon, J. I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nature Immunol. 4, 269–273 (2003).
Cash, H. L., Whitham, C. V., Behrendt, C. L. & Hooper, L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006).
Cash, H. L., Whitham, C. V. & Hooper, L. V. Refolding, purification, and characterization of human and murine RegIII proteins expressed in Escherichia coli. Protein Expr. Purif. 48, 151–159 (2006).
Ayabe, T. et al. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nature Immunol. 1, 113–118 (2000).
Dessein, R. et al. Toll-like receptor 2 is critical for induction of Reg3β expression and intestinal clearance of Yersinia pseudotuberculosis. Gut 58, 771–776 (2009).
Kobayashi, K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005).
Brandl, K., Plitas, G., Schnabl, B., DeMatteo, R. P. & Pamer, E. G. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. J. Exp. Med. 204, 1891–1900 (2007).
Vaishnava, S., Behrendt, C. L., Ismail, A. S., Eckmann, L. & Hooper, L. V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl Acad. Sci. USA 105, 20858–20863 (2008). Using MYD88-deficient mice, reference 108 shows that TLR signalling is required for the production of antimicrobial lectins, which limit infection and dissemination of Listeria monocytogenes . Reference 109 indicates that TLR signalling by Paneth cells themselves is required for the production of REG3γ and other antimicrobial peptides.
Brandl, K. et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455, 804–807 (2008).
Macpherson, A. J., McCoy, K. D., Johansen, F. E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008).
He, B. et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 (2007). This study shows that activation of TLRs in IECs induces the expression of APRIL, which promotes local (lamina propria) class switching of IgA1 to IgA2.
Castigli, E. et al. TACI and BAFF-R. mediate isotype switching in B cells. J. Exp. Med. 201, 35–39 (2005).
Shang, L. et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology 135, 529–538 (2008).
Chieppa, M., Rescigno, M., Huang, A. Y. & Germain, R. N. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 203, 2841–2852 (2006).
Nenci, A. et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Kim, J. Y., Kajino-Sakamoto, R., Omori, E., Jobin, C. & Ninomiya-Tsuji, J. Intestinal epithelial-derived TAK1 signaling is essential for cytoprotection against chemical-induced colitis. PLoS ONE 4, e4561 (2009).
Kajino-Sakamoto, R. et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J. Immunol. 181, 1143–1152 (2008).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007).
Zaph, C. et al. Commensal-dependent expression of IL-25 regulates the IL-23–IL-17 axis in the intestine. J. Exp. Med. 205, 2191–2198 (2008).
Zeuthen, L. H., Fink, L. N. & Frokiaer, H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-β. Immunology 123, 197–208 (2008).
Troy, A. E. et al. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J. Immunol. 183, 2037–2044 (2009).
Taylor, B. C. et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206, 655–667 (2009).
Butler, M. et al. Modulation of dendritic cell phenotype and function in an in vitro model of the intestinal epithelium. Eur. J. Immunol. 36, 864–874 (2006).
Dignass, A., Lynch-Devaney, K., Kindon, H., Thim, L. & Podolsky, D. K. Trefoil peptides promote epithelial migration through a transforming growth factor β-independent pathway. J. Clin. Invest. 94, 376–383 (1994).
Mashimo, H., Wu, D. C., Podolsky, D. K. & Fishman, M. C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274, 262–265 (1996).
Podolsky, D. K., Gerken, G., Eyking, A. & Cario, E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 137, 209–220 (2009). This paper indicates that the production of TFF3 depends on TLR2 signalling and protects against colitis.
Segditsas, S. & Tomlinson, I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25, 7531–7537 (2006).
Su, L. K. et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 (1992).
Powell, S. M. et al. APC mutations occur early during colorectal tumorigenesis. Nature 359, 235–237 (1992).
Dove, W. F. et al. Intestinal neoplasia in the ApcMin mouse: independence from the microbial and natural killer (beige locus) status. Cancer Res. 57, 812–814 (1997).
Rakoff-Nahoum, S. & Medzhitov, R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 317, 124–127 (2007). Using a mouse model of APC-dependent colorectal cancer, this study suggests that TLR signalling is necessary for growth of intestinal tumours.
Phelps, R. A. et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell 137, 623–634 (2009).
Rutter, M. et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 126, 451–459 (2004).
Clevers, H. At the crossroads of inflammation and cancer. Cell 118, 671–674 (2004).
Yang, G. Y., Taboada, S. & Liao, J. Inflammatory bowel disease: a model of chronic inflammation-induced cancer. Methods Mol. Biol. 511, 193–233 (2009).
Garlanda, C. et al. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 67, 6017–6021 (2007).
Rhee, S. H., Im, E. & Pothoulakis, C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology 135, 518–528 (2008).
Huang, B. et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 65, 5009–5014 (2005).
Sun, Q., Liu, Q., Zheng, Y. & Cao, X. Rapamycin suppresses TLR4-triggered IL-6 and PGE2 production of colon cancer cells by inhibiting TLR4 expression and NF-κB activation. Mol. Immunol. 45, 2929–2936 (2008).
Tanabe, H. et al. Mouse Paneth cell secretory responses to cell surface glycolipids of virulent and attenuated pathogenic bacteria. Infect. Immun. 73, 2312–2320 (2005).
Ortega-Cava, C. F. et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J. Immunol. 170, 3977–3985 (2003).
Gomariz, R. P. et al. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J. Leukoc. Biol. 78, 491–502 (2005).
Ruiz, P. A., Shkoda, A., Kim, S. C., Sartor, R. B. & Haller, D. IL-10 gene-deficient mice lack TGF-β/Smad signaling and fail to inhibit proinflammatory gene expression in intestinal epithelial cells after the colonization with colitogenic Enterococcus faecalis. J. Immunol. 174, 2990–2999 (2005).
Singh, J. C. et al. Toll-like receptor-mediated responses of primary intestinal epithelial cells during the development of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G514–G524 (2005).
Gopal, R., Birdsell, D. & Monroy, F. Regulation of Toll-like receptors in intestinal epithelial cells by stress and Toxoplasma gondii infection. Parasite Immunol. 30, 563–576 (2008).
Furrie, E., Macfarlane, S., Thomson, G. & Macfarlane, G. T. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology 115, 565–574 (2005).
Naik, S., Kelly, E. J., Meijer, L., Pettersson, S. & Sanderson, I. R. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J. Pediatr. Gastroenterol. Nutr. 32, 449–453 (2001).
Zhou, R. B., Wei, H. M., Sun, R. & Tian, Z. G. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J. Immunol. 178, 4548–4556 (2007).
Ohkawara, T. et al. Regulation of Toll-like receptor 4 expression in mouse colon by macrophage migration inhibitory factor. Histochem. Cell Biol. 125, 575–582 (2006).
Reigstad, C., Lundén, G., Felin, J. & Bäckhed, F. Regulation of serum amyloid A3 (SAA3) in mouse colonic epithelium and adipose tissue by the intestinal microbiota. PLoS ONE 4, e5842 (2009).
Neal, M. D. et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 176, 3070–3079 (2006).
Tyrer, P., Foxwell, A. R., Cripps, A. W., Apicella, M. A. & Kyd, J. M. Microbial pattern recognition receptors mediate M-cell uptake of a Gram-negative bacterium. Infect. Immun. 74, 625–631 (2006).
Bashir, M. E., Louie, S., Shi, H. N. & Nagler-Anderson, C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J. Immunol. 172, 6978–6987 (2004).
Abreu, M. T. et al. TLR signaling at the intestinal epithelial interface. J. Endotoxin Res. 9, 322–330 (2003).
Miyamoto, Y., Iimura, M., Kaper, J., B., Torres, A., G. & Kagnoff, M., F. Role of Shiga toxin versus H7 flagellin in enterohaemorrhagic Escherichia coli signalling of human colon epithelium in vivo. Cell. Microbiol. 8, 869–879 (2006).
Rumio, C. et al. Degranulation of paneth cells via Toll-like receptor 9. Am. J. Pathol. 165, 373–381 (2004).
Acknowledgements
I thank L. Hayes for her assistance in the preparation of this review.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary information S1 (Table)
Expression of Toll-like receptors in cell lines (PDF 143 kb)
Related links
Related links
FURTHER INFORMATION
National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) Program
Glossary
- Lamina propria
-
The layer of mucosal tissue directly under the mucosal epithelial cell surface of the gastrointestinal tract, in which mucosal effector immune cells reside.
- Goblet cells
-
Mucus-producing cells found in the epithelial cell lining of the intestine and lungs.
- Enteroendocrine cells
-
Specialized endocrine cells of the gastrointestinal tract that differentiate from pluripotent stem cells. They are characterized by their ability to produce hormones such as serotonin, somatostatin, motilin, cholecystokinin, vasoactive intestinal peptide and enteroglucagon.
- Paneth cells
-
Specialized cells that are generally only found at the base of small intestinal crypts, although they can also be seen in the human colon in chronic inflammatory conditions. Paneth cells produce antimicrobial peptides or lectins, including REG3 proteins and defensins.
- Tight junction
-
A belt-like region of adhesion between adjacent epithelial or endothelial cells that regulates paracellular flux. Tight junction proteins include the integral membrane proteins occludin and claudin, in association with cytoplasmic zonula occludens proteins.
- Laser capture microdissection
-
A method used to isolate specific subpopulations of cells under direct microscopy of the tissue. A laser is used to separate the desired cells from the tissue on a microscope slide and the cells of interest are then captured for subsequent analysis.
- Crypts
-
Tubular invaginations of the intestinal epithelium. At the base of the crypts, there are Paneth cells, which produce bactericidal defensins, and stem cells, which continuously divide and are the source of all intestinal epithelial cells.
- CpG-containing oligodeoxynucleotides
-
Synthetic DNA sequences that include a cytosine–guanosine sequence and certain flanking nucleotides, which have been found to induce innate immune responses through interaction with TLR9.
- Crohn's disease
-
A form of chronic inflammatory bowel disease that can affect the entire gastrointestinal tract but is most common in the colon and terminal ileum. It is characterized by transmural inflammation, strictures and granuloma formation, and is thought to result from an abnormal T cell-mediated immune response to commensal bacteria. Symptoms include abdominal pain, rectal bleeding, diarrhoea and weight loss.
- Villi
-
Projections into the lumen that have an outer layer that mainly consists of mature, absorptive enterocytes and mucus-secreting goblet cells.
- Follicle-associated epithelium
-
(FAE). The epithelium that overlies mucosal lymphoid tissues, such as the Peyer's patches and the isolated lymphoid follicles in the intestine. Lymphoid tissues induce the differentiation of normal intestinal epithelium into FAE, which is specialized in antigen capture and transport.
- WNT proteins
-
Glycoproteins related to the Drosophila melanogaster protein Wingless, a ligand that regulates the temporal and spatial development of the embryo. WNT-mediated signalling has been shown to regulate cell fate determination, proliferation, adhesion, migration and polarity during development. WNT and its downstream signalling molecules also have been implicated in tumorigenesis and have causative roles in human colon cancers.
- Cross-tolerance
-
A state of unresponsiveness in which cells exposed to a TLR ligand show tolerance to subsequent challenge with the same stimulus and also to subsequent challenges with other stimuli that signal through one or more different TLRs.
- Septic shock
-
A systemic response to severe bacterial infections or Gram-negative bacterial endotoxins (such as LPS) that leads to a hyperactive and dysregulated network of inflammatory cytokines, affecting vascular permeability, cardiac function and metabolic balance, which leads to tissue necrosis, multiple-organ failure and death.
- Necrotizing enterocolitis
-
(NEC). A gastrointestinal disease predominantly affecting premature low-birth-weight infants. NEC involves infection and inflammation that causes destruction of the intestine. Although the pathophysiology of NEC is not completely defined, increasing evidence indicates that immaturity of intestinal innate immune function of the premature gut, characterized by over-exuberant IL-8 responses of intestinal epithelial cells to LPS, is a key factor.
Rights and permissions
About this article
Cite this article
Abreu, M. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10, 131–144 (2010). https://doi.org/10.1038/nri2707
Issue Date:
DOI: https://doi.org/10.1038/nri2707
This article is cited by
-
Increased Intestinal Permeability: An Avenue for the Development of Autoimmune Disease?
Exposure and Health (2024)
-
Dysfunctional TLR1 reduces the therapeutic efficacy of chemotherapy by attenuating HMGB1-mediated antitumor immunity in locally advanced colorectal cancer
Scientific Reports (2023)
-
Gut Microbiota Resilience Mechanisms Against Pathogen Infection and its Role in Inflammatory Bowel Disease
Current Clinical Microbiology Reports (2023)
-
Microencapsulate Probiotics (MP) Promote Growth Performance and Inhibit Inflammatory Response in Broilers Challenged with Salmonella typhimurium
Probiotics and Antimicrobial Proteins (2023)
-
Role of mucosal immunity and epithelial–vascular barrier in modulating gut homeostasis
Internal and Emergency Medicine (2023)