Key Points
-
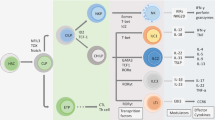
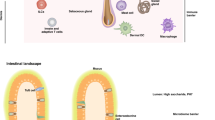
Human skin and the immune cells that it contains provide essential protection of the human body from injury and infection.
-
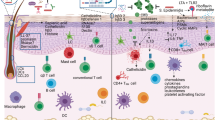
Keratinocytes are a first line of defence and sense danger through alert systems such as the inflammasome and Toll-like receptors. They mediate an inflammatory response by secreting pro-inflammatory cytokines.
-
A subpopulation of CD103+ dendritic cells is strategically positioned for cross-presentation of skin-tropic pathogens.
-
Recent data have highlighted a key role of tissue-resident rather than circulating T cells in skin homeostasis and pathology.
-
Important lessons for human skin immunology have been learnt from immunologically targeted therapies in inflammatory skin disorders such as psoriasis.
Abstract
Human skin and its immune cells provide essential protection of the human body from injury and infection. Recent studies reinforce the importance of keratinocytes as sensors of danger through alert systems such as the inflammasome. In addition, newly identified CD103+ dendritic cells are strategically positioned for cross-presentation of skin-tropic pathogens and accumulating data highlight a key role of tissue-resident rather than circulating T cells in skin homeostasis and pathology. This Review focuses on recent progress in dissecting the functional role of skin immune cells in skin disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Streilein, J. W. Skin-associated lymphoid tissues (SALT): origins and functions. J. Invest. Dermatol. 80, 12S–16S (1983). This is a milestone paper introducing the concept of SALT for the first time and describing the skin in conjunction with draining lymph nodes as an immune-competent organ.
Bos, J. D. & Kapsenberg, M. L. The skin immune system (SIS): its cellular constituents and their interactions. Immunol. Today 7, 235–240 (1986).
Stingl, G. & Bergstresser, P. R. Dendritic cells: a major story unfolds. Immunol. Today 16, 330–333 (1995).
Nickoloff, B. J. (ed.) Dermal Immune System (CRC, Boca Raton, 1993).
Proksch, E., Brandner, J. M. & Jensen, J. M. The skin: an indispensable barrier. Exp. Dermatol. 17, 1063–1072 (2008).
Krueger, G. G. & Stingl, G. Immunology/inflammation of the skin — a 50-year perspective. J. Invest. Dermatol. 92, 32S–51S (1989).
Janeway, C. A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).
Lebre, M. C. et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Invest. Dermatol. 127, 331–341 (2007).
Kalali, B. N. et al. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J. Immunol. 181, 2694–2704 (2008).
Miller, L. S. & Modlin, R. L. Human keratinocyte Toll-like receptors promote distinct immune responses. J. Invest. Dermatol. 127, 262–263 (2007).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Feldmeyer, L. et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Curr. Biol. 17, 1140–1145 (2007).
Keller, M., Ruegg, A., Werner, S. & Beer, H. D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008). References 12 and 13 are key papers showing that the inflammasome machinery is responsible for UV-induced secretion of IL-1β by human keratinocytes.
Watanabe, H. et al. Activation of the IL-1β-processing inflammasome is involved in contact hypersensitivity. J. Invest. Dermatol. 127, 1956–1963 (2007).
Gilliet, M. & Lande, R. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr. Opin. Immunol. 20, 401–407 (2008).
Lai, Y. & Gallo, R. L. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 30, 131–141 (2009).
Weaver, C. T., Hatton, R. D., Mangan, P. R. & Harrington, L. E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852 (2007).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Kolls, J. K., McCray, P. B. Jr & Chan, Y. R. Cytokine-mediated regulation of antimicrobial proteins. Nature Rev. Immunol. 8, 829–835 (2008).
Harder, J., Bartels, J., Christophers, E. & Schroder, J. M. A peptide antibiotic from human skin. Nature 387, 861 (1997).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007). A landmark paper showing that LL37 can convert inert self DNA into a potent pro-inflammatory trigger for pDC IFNα production through TLR9-dependent mechanisms, suggesting that this pathway might drive autoimmunity in the context of skin inflammation but also other autoimmune-type disorders.
Schauber, J. et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 117, 803–811 (2007).
Peric, M. et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J. Immunol. 181, 8504–8512 (2008).
Albanesi, C., Scarponi, C., Giustizieri, M. L. & Girolomoni, G. Keratinocytes in inflammatory skin diseases. Curr. Drug Targets Inflamm. Allergy 4, 329–334 (2005).
Arend, W. P., Palmer, G. & Gabay, C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223, 20–38 (2008).
Lee, P. et al. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature 458, 519–523 (2009).
Groves, R. W., Mizutani, H., Kieffer, J. D. & Kupper, T. S. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1α in basal epidermis. Proc. Natl Acad. Sci. USA 92, 11874–11878 (1995).
Blumberg, H. et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 204, 2603–2614 (2007).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature Immunol. 3, 673–680 (2002).
Dieu-Nosjean, M. C. et al. Macrophage inflammatory protein 3α is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 192, 705–718 (2000).
Nickoloff, B. J. & Turka, L. A. Immunological functions of non-professional antigen-presenting cells: new insights from studies of T-cell interactions with keratinocytes. Immunol. Today 15, 464–469 (1994).
Gaspari, A. A. & Katz, S. I. Induction of in vivo hyporesponsiveness to contact allergens by hapten-modified Ia+ keratinocytes. J. Immunol. 147, 4155–4161 (1991).
Nickoloff, B. J. et al. Accessory cell function of keratinocytes for superantigens. Dependence on lymphocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction. J. Immunol. 150, 2148–2159 (1993).
Black, A. P. et al. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur. J. Immunol. 37, 1485–1493 (2007).
Griffiths, C. E. & Nickoloff, B. J. Keratinocyte intercellular adhesion molecule-1 (ICAM-1) expression precedes dermal T lymphocytic infiltration in allergic contact dermatitis (Rhus dermatitis). Am. J. Pathol. 135, 1045–1053 (1989).
Kupper, T. S. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J. Invest. Dermatol. 94, 146S–150S (1990).
Luger, T. A. & Schwarz, T. Evidence for an epidermal cytokine network. J. Invest. Dermatol. 95, 100S–104S (1990).
Barker, J. N., Mitra, R. S., Griffiths, C. E., Dixit, V. M. & Nickoloff, B. J. Keratinocytes as initiators of inflammation. Lancet 337, 211–214 (1991).
Mehling, A. et al. Overexpression of CD40 ligand in murine epidermis results in chronic skin inflammation and systemic autoimmunity. J. Exp. Med. 194, 615–628 (2001).
Zenz, R. et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437, 369–375 (2005).
Pasparakis, M. et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417, 861–866 (2002).
Eckmann, L. et al. Opposing functions of IKKβ during acute and chronic intestinal inflammation. Proc. Natl Acad. Sci. USA 105, 15058–15063 (2008).
Sano, S. et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nature Med. 11, 43–49 (2005).
Oppenheim, D. E. et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nature Immunol. 6, 928–937 (2005).
Strid, J. et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nature Immunol. 9, 146–154 (2008). This paper provides insights into the early phases of tissue immunosurveillance, establishing that upregulation of the NKG2D ligand RAE1α can promote considerable reorganization of the skin immune compartment.
Bursch, L. S. et al. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204, 3147–3156 (2007).
Ginhoux, F. et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204, 3133–3146 (2007).
Poulin, L. F. et al. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 204, 3119–3131 (2007). References 46–48 describe a newly discovered population of skin DCs: langerin+ non-Langerhans cells.
Nagao, K. et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl Acad. Sci. USA 106, 3312–3317 (2009).
Merad, M., Ginhoux, F. & Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nature Rev. Immunol. 8, 935–947 (2008).
Romani, N. et al. Epidermal Langerhans cells — changing views on their function in vivo. Immunol. Lett. 106, 119–125 (2006).
Hunger, R. E. et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J. Clin. Invest. 113, 701–708 (2004).
Klechevsky, E. et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity 29, 497–510 (2008).
Stoitzner, P. et al. Langerhans cells cross-present antigen derived from skin. Proc. Natl Acad. Sci. USA 103, 7783–7788 (2006).
Waithman, J. et al. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J. Immunol. 179, 4535–4541 (2007).
Bedoui, S. et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nature Immunol. 10, 488–495 (2009). This paper establishes that CD103+ DCs are the key cross-presenting APCs in the skin.
Schuler, G. & Steinman, R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 161, 526–546 (1985). This key paper describes the concept of immature DCs (specialized for antigen processing) and mature DCs (specialized for antigen presentation) for the first time, using the example of Langerhans cells.
Grabbe, S., Steinbrink, K., Steinert, M., Luger, T. A. & Schwarz, T. Removal of the majority of epidermal Langerhans cells by topical or systemic steroid application enhances the effector phase of murine contact hypersensitivity. J. Immunol. 155, 4207–4217 (1995).
Kaplan, D. H., Kissenpfennig, A. & Clausen, B. E. Insights into Langerhans cell function from Langerhans cell ablation models. Eur. J. Immunol. 38, 2369–2376 (2008).
Allan, R. S. et al. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301, 1925–1928 (2003).
Zhao, X. et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197, 153–162 (2003).
Wollenberg, A. et al. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J. Invest. Dermatol. 118, 327–334 (2002).
Bieber, T. The pro- and anti-inflammatory properties of human antigen-presenting cells expressing the high affinity receptor for IgE (FcɛRI). Immunobiology 212, 499–503 (2007).
Guttman-Yassky, E. et al. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J. Allergy Clin. Immunol. 119, 1210–1217 (2007).
Kissenpfennig, A. et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22, 643–654 (2005).
Fukunaga, A., Khaskhely, N. M., Sreevidya, C. S., Byrne, S. N. & Ullrich, S. E. Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J. Immunol. 180, 3057–3064 (2008).
Auffray, C., Sieweke, M. H. & Geissmann, F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692 (2009).
Lopez-Bravo, M. & Ardavin, C. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity 29, 343–351 (2008).
Zaba, L. C., Fuentes-Duculan, J., Steinman, R. M., Krueger, J. G. & Lowes, M. A. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J. Clin. Invest. 117, 2517–2525 (2007).
Ochoa, M. T., Loncaric, A., Krutzik, S. R., Becker, T. C. & Modlin, R. L. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J. Invest. Dermatol. 128, 2225–2231 (2008).
Nestle, F. O. & Nickoloff, B. J. Deepening our understanding of immune sentinels in the skin. J. Clin. Invest. 117, 2382–2385 (2007).
Nestle, F. O., Zheng, X. G., Thompson, C. B., Turka, L. A. & Nickoloff, B. J. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J. Immunol. 151, 6535–6545 (1993).
Lenz, A., Heine, M., Schuler, G. & Romani, N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J. Clin. Invest. 92, 2587–2596 (1993). References 72 and 73 provided the first functional characterization of human dermal DCs.
Larregina, A. T. et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nature Immunol. 2, 1151–1158 (2001).
Angel, C. E. et al. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int. Immunol. 19, 1271–1279 (2007).
Boyman, O. et al. Activation of dendritic antigen-presenting cells expressing common heat shock protein receptor CD91 during induction of psoriasis. Br. J. Dermatol. 152, 1211–1218 (2005).
Serbina, N. V., Salazar-Mather, T. P., Biron, C. A., Kuziel, W. A. & Pamer, E. G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 (2003).
Lowes, M. A. et al. Increase in TNF-α and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl Acad. Sci. USA 102, 19057–19062 (2005).
Blanco, P., Palucka, A. K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001).
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202, 135–143 (2005). References 79 and 80 establish the functional role of pDCs and their production of IFNα in the pathogenesis of immune-mediated diseases such as systemic lupus erythematosus and psoriasis.
Boyman, O., Conrad, C., Tonel, G., Gilliet, M. & Nestle, F. O. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol. 28, 51–57 (2007).
van Furth, R., Nibbering, P. H., van Dissel, J. T. & Diesselhoff-den Dulk, M. M. The characterization, origin, and kinetics of skin macrophages during inflammation. J. Invest. Dermatol. 85, 398–402 (1985).
Clark, R. A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006). This is an important paper showing that normal skin harbours a large number of skin-homing memory T cells, which are 2.8-fold more abundant than T cells circulating in the blood.
Andrew, W. & Andrew, N. V. Lymphocytes in the normal epidermis of the rat and of man. Anat. Rec. 104, 217–241 (1949).
Bos, J. D. et al. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J. Invest. Dermatol. 88, 569–573 (1987).
Foster, C. A. et al. Human epidermal T cells predominantly belong to the lineage expressing alpha/beta T cell receptor. J. Exp. Med. 171, 997–1013 (1990).
Bos, J. D. & Kapsenberg, M. L. The skin immune system: progress in cutaneous biology. Immunol. Today 14, 75–78 (1993).
Mora, J. R. et al. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201, 303–316 (2005).
Edele, F. et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J. Immunol. 181, 3745–3749 (2008).
Sigmundsdottir, H. & Butcher, E. C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nature Immunol. 9, 981–987 (2008).
Di Cesare, A., Di Meglio, P. & Nestle, F. O. The IL-23/Th17 axis in immunopathogenesis of psoriasis. J. Invest. Dermatol. 129, 1339–1350 (2009).
Di Cesare, A., Di Meglio, P. & Nestle, F. O. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J. Invest. Dermatol. 128, 2569–2571 (2008).
de Beaucoudrey, L. et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 205, 1543–1550 (2008). Studying patients with immune deficiencies, these authors describe IL-12Rβ1- and STAT3-dependent signals as key components of T H 17 cell differentiation.
Milner, J. D. et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 (2008).
Eyerich, K. et al. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J. Invest. Dermatol. 128, 2640–2645 (2008).
Duhen, T., Geiger, R., Jarrossay, D., Lanzavecchia, A. & Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nature Immunol. 10, 857–863 (2009).
Trifari, S., Kaplan, C. D., Tran, E. H., Crellin, N. K. & Spits, H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nature Immunol. 10, 864–871 (2009).
Nograles, K. E. et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 123, 1244–1252 (2009). References 96–98 describe T H 22 cells as a skin-homing T H cell population that secretes IL-22 but not IL-17 or IFNγ.
Luster, A. D., Alon, R. & von Andrian, U. H. Immune cell migration in inflammation: present and future therapeutic targets. Nature Immunol. 6, 1182–1190 (2005).
Kupper, T. S. & Fuhlbrigge, R. C. Immune surveillance in the skin: mechanisms and clinical consequences. Nature Rev. Immunol. 4, 211–222 (2004).
Boyman, O. et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199, 731–736 (2004).
Conrad, C. et al. α1β1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nature Med. 13, 836–842 (2007). References 101 and 102 both use a new psoriasis xenotransplantation model to show evidence for a functional role of tissue-resident T cells in the pathogenesis of psoriasis. They also identify the binding of VLA1 on T cells to collagen IV as a key checkpoint for T cell entry into the epidermis during inflammation.
Wakim, L. M., Waithman, J., van Rooijen, N., Heath, W. R. & Carbone, F. R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunol. 10, 524–530 (2009). Using an HSV infection model, this paper describes a unique and protective skin-resident memory T cell subset, supporting a role of tissue-resident T cells in the memory response to infection.
Woodland, D. L. & Kohlmeier, J. E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nature Rev. Immunol. 9, 153–161 (2009).
Hayday, A. & Tigelaar, R. Immunoregulation in the tissues by γδ T cells. Nature Rev. Immunol. 3, 233–242 (2003).
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23, 877–900 (2005).
Bergstresser, P. R., Sullivan, S., Streilein, J. W. & Tigelaar, R. E. Origin and function of Thy-1+ dendritic epidermal cells in mice. J. Invest. Dermatol. 85, 85S–90S (1985).
Girardi, M. Immunosurveillance and immunoregulation by γδ T cells. J. Invest. Dermatol. 126, 25–31 (2006).
Boyden, L. M. et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nature Genet. 40, 656–662 (2008).
Strid, J., Tigelaar, R. E. & Hayday, A. C. Skin immune surveillance by T cells — a new order? Semin. Immunol. 21, 110–120 (2009).
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001).
Girardi, M. et al. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J. Exp. Med. 195, 855–867 (2002).
Roberts, S. J. et al. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc. Natl Acad. Sci. USA 104, 6770–6775 (2007).
Holtmeier, W. & Kabelitz, D. γδ T cells link innate and adaptive immune responses. Chem. Immunol. Allergy 86, 151–183 (2005).
Toulon, A. et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 206, 743–750 (2009).
Agerberth, B. et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96, 3086–3093 (2000).
Nickoloff, B. J. Skin innate immune system in psoriasis: friend or foe? J. Clin. Invest. 104, 1161–1164 (1999).
Nickoloff, B. J., Bonish, B., Huang, B. B. & Porcelli, S. A. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J. Dermatol. Sci. 24, 212–225 (2000).
Bonish, B. et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-γ production by NK-T cells. J. Immunol. 165, 4076–4085 (2000).
Gober, M. D., Fishelevich, R., Zhao, Y., Unutmaz, D. & Gaspari, A. A. Human natural killer T cells infiltrate into the skin at elicitation sites of allergic contact dermatitis. J. Invest. Dermatol. 128, 1460–1469 (2008).
Gorbachev, A. V. & Fairchild, R. L. Activated NKT cells increase dendritic cell migration and enhance CD8+ T cell responses in the skin. Eur. J. Immunol. 36, 2494–2503 (2006).
Grabbe, S. & Schwarz, T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol. Today 19, 37–44 (1998).
Gober, M. D. & Gaspari, A. A. Allergic contact dermatitis. Curr. Dir. Autoimmun. 10, 1–26 (2008).
Lonsdorf, A. S. & Enk, A. H. Immunologie des allergischen kontaktekzems. Hautarzt 60, 32–41 (2009) (in German).
Martin, S. F. & Jakob, T. From innate to adaptive immune responses in contact hypersensitivity. Curr. Opin. Allergy Clin. Immunol. 8, 289–293 (2008).
Nestle, F. O., Kaplan, D. H. & Barker, J. Psoriasis. N. Engl. J. Med. 361, 496–509 (2009).
Ellis, C. N. et al. Cyclosporine improves psoriasis in a double-blind study. JAMA 256, 3110–3116 (1986).
Prinz, J. C. et al. Selection of conserved TCR VDJ rearrangements in chronic psoriatic plaques indicates a common antigen in psoriasis vulgaris. Eur. J. Immunol. 29, 3360–3368 (1999).
Austin, L. M., Ozawa, M., Kikuchi, T., Walters, I. B. & Krueger, J. G. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-γ, interleukin-2, and tumor necrosis factor-α, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Invest. Dermatol. 113, 752–759 (1999).
Valdimarsson, H., Baker, B. S., Jonsdottir, I., Powles, A. & Fry, L. Psoriasis: a T-cell-mediated autoimmune disease induced by streptococcal superantigens? Immunol. Today 16, 145–149 (1995).
Lowes, M. A., Bowcock, A. M. & Krueger, J. G. Pathogenesis and therapy of psoriasis. Nature 445, 866–873 (2007).
Capon, F., Munro, M., Barker, J. & Trembath, R. Searching for the major histocompatibility complex psoriasis susceptibility gene. J. Invest. Dermatol. 118, 745–751 (2002).
de Cid, R. et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nature Genet. 41, 211–215 (2009).
Nair, R. P. et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nature Genet. 41, 199–204 (2009). A comprehensive whole-genome scan of psoriasis confirming association with gene variants in the IL-23 pathway and pointing towards new gene variants involved in the NF-κB pathway.
Cargill, M. et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 80, 273–290 (2007).
Capon, F. et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum. Genet. 122, 201–206 (2007). References 136 and 137 are the first description of an association of psoriasis with IL23R gene variants in whole-genome association studies.
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Acknowledgements
We apologize to all the authors whose work could not be discussed and cited owing to space limitations. We thank R. Trembath, A. Hayday, J. Barker and F. Geissmann for discussions. We acknowledge support by the following grant funding bodies: Wellcome Trust Programme GR078173MA, National Institute of Health RO1AR040065, National Insitute for Health Research Comprehensive Biomedical Research Centre at Guy's and St. Thomas' Hospital and King's College London, Medical Research Council UK Programme G0601387, and Dunhill Medical Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Langerhans cell
-
A type of dendritic cell that is resident in the epidermal layer of the skin.
- Keratinocytes
-
The major cell type of the epidermis, constituting more than 90% of epidermal cells. Keratinocytes form an effective barrier against the entry of foreign matter and infectious agents into the body and minimize moisture loss.
- γδ T cells
-
T cells that express heterodimers consisting of the γ- and δ-chains of the T cell receptor (TCR). They enter tissues such as the gut and skin without priming in lymphoid tissues, express limited or invariant TCRs and display a 'pseudo-memory' T cell phenotype allowing them to respond rapidly to antigen challenge.
- Hapten
-
A molecule that can bind antibody but is thought not to elicit an immune response itself. Antibodies that are specific for a hapten can be generated when the hapten is chemically linked to a protein carrier that can elicit a T cell response.
- Allergic contact dermatitis
-
A cutaneous inflammatory condition caused by a T cell-mediated hypersensitivity to defined allergens.
- β-defensins and cathelicidins
-
Members of a family of small antimicrobial polypeptides that are abundant in neutrophils and epithelial cells. They contribute to host defence by disrupting the cytoplasmic membrane of microorganisms such as Escherichia coli or Candida albicans.
- Tolerance
-
Denotes lymphocyte non-responsiveness to antigen, but implies an active process, not simply a passive lack of response.
- LL37
-
A member of the cathelicidin family of antimicrobial peptides. LL37 has been proposed to have a specific role in psoriasis pathogenesis, contributing to breaking the tolerance to self DNA.
- Graft-versus-host disease
-
(GVHD). A disease that results from donor allogeneic T cells that are transferred along with an allograft (such as a bone marrow, liver or gut allograft) attacking target recipient organs or tissues (such as the skin or gut). GVHD occurs in graft recipients that cannot eliminate the host-reactive donor T cells owing to immunosuppression, immunological immaturity or tolerance.
- T cell anergy
-
A state of T cell unresponsiveness to stimulation with antigen. It can be induced by stimulation with a large amount of specific antigen in the absence of the engagement of co-stimulatory molecules.
- Plasmacytoid DC
-
A dendritic cell (DC) that lacks myeloid markers such as CD11c and CD33 but expresses high levels of HLA-DR and CD123. These cells produce high levels of type I interferon after activation (for example, when stimulated through Toll-like receptors).
- Cross-presentation
-
The initiation of a CD8+ T cell response to an antigen that is not present within antigen-presenting cells (APCs). This exogenous antigen must be taken up by APCs and then re-routed to the MHC class I pathway of antigen presentation.
- Birbeck granules
-
Membrane-bound rod- or tennis racket-shaped structures with a central linear density, found in the cytoplasm of Langerhans cells. Their formation is induced by langerin.
- Contact hypersensitivity
-
The inflammatory reaction that occurs after the first exposure to a 'sensitizer' hapten or antigen. This step requires dendritic cell migration to lymph nodes to prime contact-antigen-specific T cells.
- Langerhans cell-deficient mice
-
Two main Langerhans cell-deficient mouse models have been developed using diphtheria toxin ablation. One model, in which diphtheria toxin receptor is constitutively expressed under the control of the human langerin promoter, shows selective depletion of langerin+ epidermal Langerhans cells. The other model, which uses the mouse langerin promoter, displays conditional depletion of all langerin+ DCs, including Langerhans cells in the epidermis, langerin+ DCs in the dermis and langerin+ DCs in lymph nodes.
- Alternatively activated macrophage
-
A macrophage stimulated by interleukin-4 (IL-4) or IL-13 that expresses arginase 1, mannose receptor CD206 and IL-4 receptor-α. There may be pathogen-associated molecular patterns expressed by helminths that can also drive alternative activation of macrophages.
- Invariant NKT cell
-
A cell type thought to be particularly important in bridging innate and adaptive immunity. iNKT cells are typified by a capacity for self-recognition and rapid release of cytokines such as interferon-γ.
Rights and permissions
About this article
Cite this article
Nestle, F., Di Meglio, P., Qin, JZ. et al. Skin immune sentinels in health and disease. Nat Rev Immunol 9, 679–691 (2009). https://doi.org/10.1038/nri2622
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2622
This article is cited by
-
Safety of applying influenza-antigen-coated microneedles to rat skin and the antigen specific immune response in vivo
Journal of Pharmaceutical Investigation (2024)
-
Staphylococcus warneri strain XSB102 exacerbates psoriasis and promotes keratinocyte proliferation in imiquimod-induced psoriasis-like dermatitis mice
Archives of Microbiology (2024)
-
New insights into the impact of microbiome on horizontal and vertical transmission of a tick-borne pathogen
Microbiome (2023)
-
Current status of skin cancers with a focus on immunology and immunotherapy
Cancer Cell International (2023)
-
Opportunities and challenges in the diagnostic utility of dermal interstitial fluid
Nature Biomedical Engineering (2023)