Key Points
-
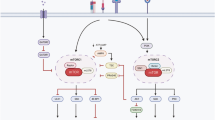
The atypical serine/threonine protein kinase mammalian target of rapamycin (mTOR) has an important role in the modulation of both innate and adaptive immune responses. A complex formed between the immunosuppressive drug rapamycin and the immunophilin FK506-binding protein 1A, 12 kDA (FKBP12) inhibits mTOR kinase activity.
-
mTOR functions in at least two multi-protein complexes: mTOR complex 1 (mTORC1) and mTORC2. mTOR in mTORC1 is highly sensitive to inhibition by rapamycin, whereas mTOR in mTORC2 is resistant to rapamycin. mTORC1 regulates cell growth downstream of phosphoinositide 3-kinase–AKT signalling, in which active mTORC1 phosphorylates S6 kinase (S6K1) and the eukaryotic translation initiation factor-binding protein 1 (EIF4EBP1). Both of these activities promote mRNA translation and cell growth.
-
Rapamycin exerts many effects on the differentiation and function of professional antigen-presenting cells (APCs). mTOR inhibition by rapamycin impedes antigen uptake and can modulate antigen presentation by dendritic cells (DCs); its differential effects on cytokine production and chemokine receptor expression by DCs regulate interactions between innate and adaptive immune cells.
-
Recent findings have shed light on previously unappreciated effects of mTOR inhibition on T cells. Rapamycin induces thymic involution, whereas the ontogeny of naturally occurring regulatory T (TReg) cells seems to be less affected. During conventional T cell activation, rapamycin-mediated mTOR inhibition blocks cell cycle progression and can sequester activated T cells in secondary lymphoid tissues. By contrast, rapamycin causes an increase in the frequency of FOXP3 (forkhead box P3)+ T cells, reflecting both the ability of TReg cells to proliferate in the presence of rapamycin and the promotion of FOXP3 expression in peripheral T cells that are then converted into modulators of immune reactivity.
-
mTOR inhibition is a promising therapeutic strategy to prevent rejection in transplantation and for autoimmune disease. Differential effects of rapamycin on T cells and TReg cells (both naturally occurring and inducible) favour its ability to promote tolerance in tolerance-enhancing protocols. In addition, adoptively transferred rapamycin-conditioned APCs inhibit organ allograft rejection and graft-versus-host disease following haematopoietic cell transplantation.
-
Ongoing and future areas of enquiry, which could prove fruitful, include distinguishing the role of mTORC1 and mTORC2 in the regulation of immune responses and tolerance, investigating the role of the mTOR–survivin–aurora B complex in T cell activation and ascertaining the mechanisms that determine TReg cell resistance to rapamycin and mTOR-mediated regulation of FOXP3 expression, as well as their relevance to therapy.
Abstract
The potent immunosuppressive action of rapamycin is commonly ascribed to inhibition of growth factor-induced T cell proliferation. However, it is now evident that the serine/threonine protein kinase mammalian target of rapamycin (mTOR) has an important role in the modulation of both innate and adaptive immune responses. mTOR regulates diverse functions of professional antigen-presenting cells, such as dendritic cells (DCs), and has important roles in the activation of effector T cells and the function and proliferation of regulatory T cells. In this Review, we discuss our current understanding of the mTOR pathway and the consequences of mTOR inhibition, both in DCs and T cells, including new data on the regulation of forkhead box P3 expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vezina, C., Kudelski, A. & Sehgal, S. N. Rapamycin (AY-22, 989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 28, 721–726 (1975).
Saunders, R. N., Metcalfe, M. S. & Nicholson, M. L. Rapamycin in transplantation: a review of the evidence. Kidney Int. 59, 3–16 (2001).
Eisen, H. J. et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N. Engl. J. Med. 349, 847–858 (2003).
Armand, P. et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J. Clin. Oncol. 26, 5767–5774 (2008).
Sabatini, D. M. mTOR and cancer: insights into a complex relationship. Nature Rev. Cancer 6, 729–734 (2006).
Roiron, C., Sanchez, P., Bouzamondo, A., Lechat, P. & Montalescot, G. Drug eluting stents: an updated meta-analysis of randomised controlled trials. Heart 92, 641–649 (2006).
Heitman, J., Movva, N. R. & Hall, M. N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253, 905–909 (1991).
Sehgal, S. N. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 35, 7S–14S (2003).
Brown, E. J. et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369, 756–758 (1994).
Chiu, M. I., Katz, H. & Berlin, V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc. Natl Acad. Sci. USA 91, 12574–12578 (1994).
Sabatini, D. M., Erdjument-Bromage, H., Lui, M., Tempst, P. & Snyder, S. H. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78, 35–43 (1994).
Gangloff, Y. G. et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol. Cell. Biol. 24, 9508–9516 (2004).
Guertin, D. A. et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt–FOXO and PKCα, but not S6K1. Dev. Cell 11, 859–871 (2006).
Wullschleger, S., Loewith, R. & Hall, M. N. TOR signaling in growth and metabolism. Cell 124, 471–484 (2006).
Thoreen, C. C. et al. An ATP-competitive mTOR inhibitor reveals rapamycin-insensitive functions of mTORC1. J. Biol. Chem. 284, 8023–8032 (2009).
Sarbassov, D. D. et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 (2006).
Zeng, Z. et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood 109, 3509–3512 (2007).
Sarbassov, D. D., Ali, S. M. & Sabatini, D. M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17, 596–603 (2005).
Yang, Q. & Guan, K. L. Expanding mTOR signaling. Cell Res. 17, 666–681 (2007).
Potter, C. J., Pedraza, L. G. & Xu, T. Akt regulates growth by directly phosphorylating Tsc2. Nature Cell Biol. 4, 658–665 (2002).
Staal, F. J., Luis, T. C. & Tiemessen, M. M. WNT signalling in the immune system: WNT is spreading its wings. Nature Rev. Immunol. 8, 581–593 (2008).
Inoki, K. et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 (2006).
Sarbassov, D. D. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004).
Jacinto, E. et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biol. 6, 1122–1128 (2004). References 23 and 24 establish that mTOR exists in two functionally distinct complexes: rapamycin-sensitive mTORC1 (which contains RAPTOR) and rapamycin-resistant mTORC2 (which contains rictor).
Hresko, R. C. & Mueckler, M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol. Chem. 280, 40406–40416 (2005).
Astrinidis, A. et al. Tuberin, the tuberous sclerosis complex 2 tumor suppressor gene product, regulates Rho activation, cell adhesion and migration. Oncogene 21, 8470–8476 (2002).
O'Reilly, K. E. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66, 1500–1508 (2006).
Tremblay, F., Gagnon, A., Veilleux, A., Sorisky, A. & Marette, A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes. Endocrinology 146, 1328–1337 (2005).
Sun, S. Y. et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 65, 7052–7058 (2005).
Greene, M. W., Sakaue, H., Wang, L., Alessi, D. R. & Roth, R. A. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by serine 312 phosphorylation. J. Biol. Chem. 278, 8199–8211 (2003).
Harrington, L. S. et al. The TSC1–2 tumor suppressor controls insulin–PI3K signaling via regulation of IRS proteins. J. Cell. Biol. 166, 213–223 (2004).
Armstrong, J. L., Bonavaud, S. M., Toole, B. J. & Yeaman, S. J. Regulation of glycogen synthesis by amino acids in cultured human muscle cells. J. Biol. Chem. 276, 952–956 (2001).
Zhang, Q., Chen, Y., Fairchild, R. L., Heeger, P. S. & Valujskikh, A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J. Immunol. 176, 770–777 (2006).
Hackstein, H. et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood 101, 4457–4463 (2003). This was the first demonstration that rapamycin administration impairs steady-state DC generation and inhibits their maturation in vivo.
Woltman, A. M. et al. Rapamycin induces apoptosis in monocyte- and CD34-derived dendritic cells but not in monocytes and macrophages. Blood 98, 174–180 (2001).
Turnquist, H. et al. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 178, 7018–7031 (2007).
Weinstein, S. L. et al. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-β. J. Leukoc. Biol. 67, 405–414 (2000).
Schmitz, F. et al. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur. J. Immunol. 38, 2981–2992 (2008).
Rama, I. et al. Hypoxia stimulus: an adaptive immune response during dendritic cell maturation. Kidney Int. 73, 816–825 (2008).
Haddadi, A. et al. Delivery of rapamycin by PLGA nanoparticles enhances its suppressive activity on dendritic cells. J. Biomed. Mater. Res. A 84, 885–898 (2008).
Das, S., Haddadi, A., Veniamin, S. & Samuel, J. Delivery of rapamycin-loaded nanoparticle down regulates ICAM-1 expression and maintains an immunosuppressive profile in human CD34+ progenitor-derived dendritic cells. J. Biomed. Mater. Res. A 85, 983–992 (2008).
Jhunjhunwala, S., Raimondi, G., Thomson, A. W. & Little, S. R. Delivery of rapamycin to dendritic cells using degradable microparticles. J. Control. Release 133, 191–197 (2009).
Hackstein, H., Taner, T., Logar, A. J. & Thomson, A. W. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood 100, 1084–1087 (2002).
Monti, P. et al. Rapamycin impairs antigen uptake of human dendritic cells. Transplantation 75, 137–145 (2003).
Fox, R. et al. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J. 26, 505–515 (2007).
Matsue, H. et al. Contrasting impacts of immunosuppressive agents (rapamycin, FK506, cyclosporin A, and dexamethasone) on bidirectional dendritic cell–T cell interaction during antigen presentation. J. Immunol. 169, 3555–3564 (2002).
Lee, Y. R. et al. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood 105, 3951–3955 (2005).
Imai, A. et al. Inhibition of endogenous MHC class II-restricted antigen presentation by tacrolimus (FK506) via FKBP51. Eur. J. Immunol. 37, 1730–1738 (2007).
Fassbender, M., Herter, S., Holtappels, R. & Schild, H. Correlation of dendritic cell maturation and the formation of aggregates of poly-ubiquitinated proteins in the cytosol. Med. Microbiol. Immunol. 197, 185–189 (2008).
Lelouard, H. et al. Transient aggregation of ubiquitinated proteins during dendritic cell maturation. Nature 417, 177–182 (2002).
Koehl, G. E. et al. Rapamycin protects allografts from rejection while simultaneously attacking tumors in immunosuppressed mice. Transplantation 77, 1319–1326 (2004).
Schmid, D., Pypaert, M. & Munz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 26, 79–92 (2007).
Vyas, J. M., Van der Veen, A. G. & Ploegh, H. L. The known unknowns of antigen processing and presentation. Nature Rev. Immunol. 8, 607–618 (2008).
Gannage, M. & Munz, C. Macroautophagy in immunity and tolerance. Traffic 24 Jan 2009 (doi:10.1111/j.1600–08542009.00883.x).
Kamada, Y. et al. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell. Biol. 150, 1507–1513 (2000).
Noda, T. & Ohsumi, Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 273, 3963–3966 (1998).
Jagannath, C. et al. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nature Med. 15, 267–276 (2009).
Woltman, A. M. et al. Rapamycin specifically interferes with GM-CSF signaling in human dendritic cells, leading to apoptosis via increased p27KIP1 expression. Blood 101, 1439–1445 (2003).
Sordi, V. et al. Differential effects of immunosuppressive drugs on chemokine receptor CCR7 in human monocyte-derived dendritic cells: selective upregulation by rapamycin. Transplantation 82, 826–834 (2006).
Reichardt, W. et al. Impact of mammalian target of rapamycin inhibition on lymphoid homing and tolerogenic function of nanoparticle-labeled dendritic cells following allogeneic hematopoietic cell transplantation. J. Immunol. 181, 4770–4779 (2008).
Taner, T., Hackstein, H., Wang, Z., Morelli, A. E. & Thomson, A. W. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. Am. J. Transplant. 5, 228–236 (2005). This was the first demonstration that recipient-derived myeloid DCs generated in rapamycin and pulsed with donor alloantigen can promote indefinite organ allograft survival in the absence of any other therapy.
Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Rev. Immunol. 3, 133–146 (2003).
Turnquist, H. R. et al. IL-1β-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J. Immunol. 181, 62–72 (2008).
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Brint, E. K. et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nature Immunol. 5, 373–379 (2004).
Fukao, T. et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nature Immunol. 3, 875–881 (2002).
Ohtani, M. et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood 112, 635–643 (2008).
Weichhart, T. et al. The TSC–mTOR signaling pathway regulates the innate inflammatory response. Immunity 29, 565–577 (2008). References 67 and 68 show that mTOR acts downstream of TLR4 and is a crucial regulator of pro- and anti-inflammatory cytokine production.
Yang, C. S. et al. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell. Microbiol. 8, 1158–1171 (2006).
Colina, R. et al. Translational control of the innate immune response through IRF-7. Nature 452, 323–328 (2008).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005).
Cao, W. et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K–mTOR–p70S76K pathway. Nature Immunol. 9, 1157–1164 (2008). This study provides in vitro and in vivo evidence that pDCs require the activation of mTOR to initiate IFNα production downstream of TLR9 ligation by CpG DNA or in response to viral challenge.
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Luo, H., Duguid, W., Chen, H., Maheu, M. & Wu, J. The effect of rapamycin on T cell development in mice. Eur. J. Immunol. 24, 692–701 (1994).
Damoiseaux, J. G., Defresne, M. P., Reutelingsperger, C. P. & Van Breda Vriesman, P. J. Cyclosporin-A differentially affects apoptosis during in vivo rat thymocyte maturation. Scand. J. Immunol. 56, 353–360 (2002).
Coenen, J. J. et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+CD25+ FoxP3+T cells. Bone Marrow Transplant. 39, 537–545 (2007).
Haxhinasto, S., Mathis, D. & Benoist, C. The AKT–mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 (2008). The authors show that the AKT–mTOR signalling pathway dominantly regulates TGFβ-dependent FOXP3 induction in peripheral CD4+ T cells.
Zheng, Y. & Rudensky, A. Y. Foxp3 in control of the regulatory T cell lineage. Nature Immunol. 8, 457–462 (2007).
Mondino, A. & Mueller, D. L. mTOR at the crossroads of T cell proliferation and tolerance. Semin. Immunol. 19, 162–172 (2007).
Song, J., Salek-Ardakani, S., So, T. & Croft, M. The kinases aurora B and mTOR regulate the G1–S cell cycle progression of T lymphocytes. Nature Immunol. 8, 64–73 (2007). This study shows the involvement of mTOR in a new complex containing aurora B, the activity of which is influenced by IL-2-mediated signalling, which is necessary for the G1 to S phase transition of the cell cycle.
Sinclair, L. V. et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nature Immunol. 9, 513–521 (2008).
Gilbert, K. M. & Weigle, W. O. Th1 cell anergy and blockade in G1a phase of the cell cycle. J. Immunol. 151, 1245–1254 (1993).
Boussiotis, V. A. et al. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science 266, 1039–1042 (1994).
Beverly, B., Kang, S. M., Lenardo, M. J. & Schwartz, R. H. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int. Immunol. 4, 661–671 (1992).
Allen, A. et al. The novel cyclophilin binding compound, sanglifehrin A, disassociates G1 cell cycle arrest from tolerance induction. J. Immunol. 172, 4797–4803 (2004).
Colombetti, S., Basso, V., Mueller, D. L. & Mondino, A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J. Immunol. 176, 2730–2738 (2006).
Fox, C. J., Hammerman, P. S. & Thompson, C. B. The Pim kinases control rapamycin-resistant T cell survival and activation. J. Exp. Med. 201, 259–266 (2005).
Roncarolo, M. G. & Battaglia, M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nature Rev. Immunol. 7, 585–598 (2007).
Battaglia, M. et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 177, 8338–8347 (2006). This is the first report of the decreased susceptibility of T Reg cells compared with conventional T cells to the anti-proliferative effect of rapamycin.
Zeiser, R. et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 108, 390–399 (2006).
Coenen, J. J., Koenen, H. J., van Rijssen, E., Hilbrands, L. B. & Joosten, I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood 107, 1018–1023 (2006).
Noris, M. et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J. Am. Soc. Nephrol. 18, 1007–1018 (2007).
Segundo, D. S. et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation 82, 550–557 (2006).
Battaglia, M., Stabilini, A. & Roncarolo, M. G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105, 4743–4748 (2005).
Strauss, L. et al. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J. Immunol. 178, 320–329 (2007).
Keever-Taylor, C. A. et al. Rapamycin enriches for CD4+ CD25+ CD27+ Foxp3+ regulatory T cells in ex vivo-expanded CD25-enriched products from healthy donors and patients with multiple sclerosis. Cytotherapy 9, 144–157 (2007).
Valmori, D. et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol. 177, 944–949 (2006).
Hickman, S. P., Yang, J., Thomas, R. M., Wells, A. D. & Turka, L. A. Defective activation of protein kinase C and Ras–ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J. Immunol. 177, 2186–2194 (2006).
Crellin, N. K., Garcia, R. V. & Levings, M. K. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood 109, 2014–2022 (2007).
Zeiser, R. et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood 111, 453–462 (2008).
Walsh, P. T. et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J. Clin. Invest. 116, 2521–2531 (2006).
Burchill, M. A., Yang, J., Vang, K. B. & Farrar, M. A. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol. Lett. 114, 1–8 (2007).
Zeiser, R. & Negrin, R. S. Interleukin-2 receptor downstream events in regulatory T cells: implications for the choice of immunosuppressive drug therapy. Cell Cycle 7, 458–462 (2008).
Basu, S., Golovina, T., Mikheeva, T., June, C. H. & Riley, J. L. Cutting Edge: Foxp3-mediated induction of Pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J. Immunol. 180, 5794–5798 (2008).
Kretschmer, K., Apostolou, I., Jaeckel, E., Khazaie, K. & von Boehmer, H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol. Rev. 212, 163–169 (2006).
Tone, Y. et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature Immunol. 9, 194–202 (2008).
Song, K., Wang, H., Krebs, T. L. & Danielpour, D. Novel roles of Akt and mTOR in suppressing TGF-β/ALK5-mediated Smad3 activation. EMBO J. 25, 58–69 (2006).
Sauer, S. et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl Acad. Sci. USA 105, 7797–7802 (2008). References 106 and 108 provide complementary insights into the molecular mechanisms by which TCR signalling regulates the expression of FOXP3 in peripheral T cells.
Lio, C. W. & Hsieh, C. S. A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 (2008).
Burchill, M. A. et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28, 112–121 (2008).
Passerini, L. et al. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int. Immunol. 20, 421–431 (2008).
Tao, R. et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nature Med. 13, 1299–1307 (2007). This report shows that combined pharmacological interference with mTOR signalling and with the epigenetic control of FOXP3 expression (histone acetylation) promotes the upregulation of FOXP3 expression in peripheral T cells.
Augustine, J. J., Bodziak, K. A. & Hricik, D. E. Use of sirolimus in solid organ transplantation. Drugs 67, 369–391 (2007).
Li, X. C. et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nature Med. 7, 114–118 (2001).
Li, Y. et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature Med. 5, 1298–1302 (1999).
Li, Y., Zheng, X. X., Li, X. C., Zand, M. S. & Strom, T. B. Combined costimulation blockade plus rapamycin but not cyclosporine produces permanent engraftment. Transplantation 66, 1387–1388 (1998).
Blaha, P. et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood 101, 2886–2893 (2003). References 115 and 117 show that mTOR inhibition, unlike calcineurin inhibition, does not block the induction of experimental transplant tolerance.
Gao, W. et al. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am. J. Transplant. 7, 1722–1732 (2007).
Battaglia, M. et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes 55, 40–49 (2006).
Uppaluri, R. et al. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation 86, 137–147 (2008).
Ueno, T. et al. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J. Clin. Invest. 118, 742–751 (2008).
Antin, J. H. et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood 102, 1601–1605 (2003).
Cutler, C. et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood 109, 3108–3114 (2007).
Raimondi, G. et al. Induction of heart transplant tolerance by post-transplant administration of allo-Ag specific Treg and rapamycin. Am. Transplant Congress 8, 249 (2008).
Carlson, R. P., Hartman, D. A., Ochalski, S. J., Zimmerman, J. L. & Glaser, K. B. Sirolimus (rapamycin, Rapamune) and combination therapy with cyclosporin A in the rat developing adjuvant arthritis model: correlation with blood levels and the effects of different oral formulations. Inflamm. Res. 47, 339–344 (1998).
Battaglia, M. et al. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes 55, 1571–1580 (2006).
Valle, A. et al. Rapamycin prevents and breaks the anti-CD3-induced tolerance in NOD mice. Diabetes 16 Jan 2009 (doi: 10.2337/db08–1432).
Monti, P. et al. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes 57, 2341–2347 (2008). This paper shows that rapamycin monotherapy in patients with autoimmune diabetes improves the function of naturally occurring T Reg cells.
Ramos-Barron, A. et al. Prevention of murine lupus disease in (NZBxNZW)F1 mice by sirolimus treatment. Lupus 16, 775–781 (2007).
Lui, S. L. et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrol. Dial. Transplant. 23, 2768–2776 (2008).
Montano Loza, A. J. & Czaja, A. J. Current therapy for autoimmune hepatitis. Nature Clin. Pract. Gastroenterol. Hepatol. 4, 202–214 (2007).
Massey, D. C., Bredin, F. & Parkes, M. Use of sirolimus (rapamycin) to treat refractory Crohn's disease. Gut 57, 1294–1296 (2008).
Yong, P. L., Russo, P. & Sullivan, K. E. Use of sirolimus in IPEX and IPEX-like children. J. Clin. Immunol. 28, 581–587 (2008).
Dames, S. A., Mulet, J. M., Rathgeb-Szabo, K., Hall, M. N. & Grzesiek, S. The solution structure of the FATC domain of the protein kinase target of rapamycin suggests a role for redox-dependent structural and cellular stability. J. Biol. Chem. 280, 20558–20564 (2005).
Kim, D. H. et al. GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 11, 895–904 (2003).
Chen, E. J. & Kaiser, C. A. LST8 negatively regulates amino acid biosynthesis as a component of the TOR pathway. J. Cell. Biol. 161, 333–347 (2003).
Frias, M. A. et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 16, 1865–1870 (2006).
Donahue, A. C. & Fruman, D. A. Proliferation and survival of activated B cells requires sustained antigen receptor engagement and phosphoinositide 3-kinase activation. J. Immunol. 170, 5851–5860 (2003).
Li, H. L., Davis, W. & Pure, E. Suboptimal cross-linking of antigen receptor induces Syk-dependent activation of p70S6 kinase through protein kinase C and phosphoinositol 3-kinase. J. Biol. Chem. 274, 9812–9820 (1999).
Donahue, A. C. & Fruman, D. A. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur. J. Immunol. 37, 2923–2936 (2007).
Sakata, A., Kuwahara, K., Ohmura, T., Inui, S. & Sakaguchi, N. Involvement of a rapamycin-sensitive pathway in CD40-mediated activation of murine B cells in vitro. Immunol. Lett. 68, 301–309 (1999).
Heidt, S. et al. Effects of immunosuppressive drugs on purified human B cells: evidence supporting the use of MMF and rapamycin. Transplantation 86, 1292–1300 (2008).
Aagaard-Tillery, K. M. & Jelinek, D. F. Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycin. Cell. Immunol. 156, 493–507 (1994).
Hornung, N., Raskova, J., Raska, K. Jr & Degiannis, D. Responsiveness of preactivated B cells to IL-2 and IL-6. Effect of cyclosporine and rapamycin. Transplantation 56, 985–990 (1993).
Wai, L. E., Fujiki, M., Takeda, S., Martinez, O. M. & Krams, S. M. Rapamycin, but not cyclosporine or FK506, alters natural killer cell function. Transplantation 85, 145–149 (2008).
Gourlay, W. A., Chambers, W. H., Monaco, A. P. & Maki, T. Importance of natural killer cells in the rejection of hamster skin xenografts. Transplantation 65, 727–734 (1998).
Gomez-Cambronero, J. Rapamycin inhibits GM-CSF-induced neutrophil migration. FEBS Lett. 550, 94–100 (2003).
Lehman, J. A., Calvo, V. & Gomez-Cambronero, J. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, Thr421/Ser424 kinase and a rapamycin-sensitive, m-TOR-related Thr389 kinase. J. Biol. Chem. 278, 28130–28138 (2003).
Lorne, E. et al. Participation of mTOR complex 1 in TLR2 and TLR4 induced neutrophil activation and acute lung injury. Am. J. Respir. Cell. Mol. Biol. 8 Jan 2009 (doi:10.1165/rcmb.2008–0290OC).
de Paulis, A. et al. Characterization of the anti-inflammatory effect of FK-506 on human mast cells. J. Immunol. 147, 4278–4285 (1991).
Acknowledgements
The authors' work is supported by National Institutes of Health (NIH) grants R01 AI060994, R01 AI067541 and U01 AI051698 and by the Roche Organ Transplantation Research Foundation 874,279,717 (to A.W.T.), by an American Heart Association Beginning Grant-in-Aid (to G.R.) and by NIH postdoctoral fellowship F32 AI072940 (to H.R.T.)
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Graft-versus-host disease
-
(GVHD). A disease that results from immunological attack by donor allogeneic T cells transferred with the allograft (such as bone marrow, liver or gut) of target recipient organs or tissues (such as skin or gut). GVHD occurs in graft recipients who cannot eliminate the host-reactive donor T cells owing to immunosuppression, immunological immaturity or tolerance of the recipient.
- Immunophilin
-
A peptidyl-prolyl isomerase that is targeted by certain immunosuppressive drugs (such as cyclosporin A, FK506 and rapamycin). The drug–immunophilin complex is responsible for the immunosuppressive action of the drug.
- Autophagy
-
A tightly regulated catabolic process that involves degradation of the cell's own intracellular components through the lysosomal machinery and is a normal part of cell growth, development and homeostasis.
- Small interfering RNA
-
A class of 20–25-nucleotide long double-stranded RNA molecules that are involved in the RNA interference pathway, which interferes with the expression of a specific gene. Also known as silencing RNA.
- FMS-like tyrosine kinase 3 ligand
-
(FLT3L). An endogenous cytokine that stimulates stem cell and progenitor cell proliferation through binding to the FLT3 receptor (a type III receptor tyrosine kinase member of the platelet-derived growth factor family). FLT3L administration markedly increases dendritic cell numbers in lymphoid and non-lymphoid tissues.
- Macropinocytosis
-
Invagination of the cell membrane to form a pocket that pinches off into the cell to form a vesicle filled with extracellular fluid and the molecules within it. The vesicle travels into the cytoplasm and fuses with other vesicles, such as lysosomes and endosomes.
- Calcineurin inhibitor
-
A complex of either cyclosporin A and cyclophilin, or FK506 and FKBP12, that inhibits the phosphatase activity of calcineurin.
- Minor histocompatibility antigen
-
An antigenic peptide derived from polymorphic cellular proteins (alloantigens) bound to MHC class I molecules that can cause rejection of MHC-matched grafts.
- Aggresome
-
A proteinaceous inclusion body that forms (within dendritic cells and other cells) when the cell's degradation machinery is impaired or overwhelmed under stress and that sequesters potentially toxic aggregates for subsequent clearance. Dendritic cell aggresome-like structures are known as DALIS.
- Two-signal T cell activation model
-
The concept that both the peptide–MHC complex (signal 1) and the co-stimulatory signals that are delivered by CD80 and CD86 expressed by antigen-presenting cells (signal 2) are required for T cell activation. Absence of signal 2 results in the induction of T cell anergy or deletion.
- T cell anergy
-
A state of T cell unresponsiveness to stimulation with antigen. It can be induced by stimulation with a large amount of specific antigen in the absence of co-stimulatory molecule engagement.
- T regulatory type 1 (TR1) cell
-
A subset of CD4+ regulatory T cells that secrete high levels of interleukin-10 (IL-10) and that downregulate T helper 1 (TH1) and (TH2) cell responses in vitro and in vivo through the secretion of soluble IL-10 and transforming growth factor-β.
- IPEX
-
A rare human disease that is linked to dysfunction of the transcriptional factor forkhead box P3 (FOXP3) and that is characterized by the development of autoimmunity.
Rights and permissions
About this article
Cite this article
Thomson, A., Turnquist, H. & Raimondi, G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9, 324–337 (2009). https://doi.org/10.1038/nri2546
Issue Date:
DOI: https://doi.org/10.1038/nri2546
This article is cited by
-
Chemoproteomic development of SLC15A4 inhibitors with anti-inflammatory activity
Nature Chemical Biology (2024)
-
mTOR and neuroinflammation in epilepsy: implications for disease progression and treatment
Nature Reviews Neuroscience (2024)
-
Fecal Microbiota Transplantation Alleviates Allergic Rhinitis via CD4+ T Cell Modulation Through Gut Microbiota Restoration
Inflammation (2024)
-
Interactions between genes altered during cardiotoxicity and neurotoxicity in zebrafish revealed using induced network modules analysis
Scientific Reports (2023)
-
Role of tissue markers associated with tumor microenvironment in the progression and immune suppression of oral squamous cell carcinoma
Medical Oncology (2023)