Key Points
-
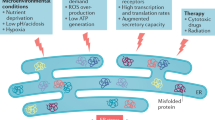
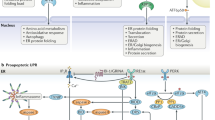
Misfolded proteins cause intracellular stress, which induces the unfolded-protein response (UPR). The UPR is a three-pronged signalling pathway that induces genes which cause cells to acquire a secretory phenotype.
-
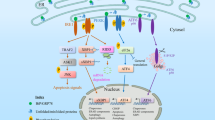
The branches of the UPR are mediated by the three endoplasmic reticulum (ER)-resident proteins: inositol-requiring 1 transmembrane kinase/endonuclease 1 (IRE1), pancreatic ER kinase (PERK), and activating transcription factor 6 (ATF6). These branches interact and have feedback mechanisms to regulate the activity of the UPR.
-
X-box binding protein 1 (XBP1), which lies downstream of IRE1, is crucial for the development of plasma cells, which secrete large amounts of immunoglobulin. The malignant variants of plasma cells, known as myeloma cells, are also dependent on an intact UPR, offering a therapeutic target for myeloma therapies. XBP1 is also important in the development and survival of dendritic cells, and suppression of XBP1 impairs the growth of a dendritic-cell tumour.
-
Abnormalities of the UPR may explain early events in the pathogenesis of autoimmunity: misfolded proteins can be immunogenic, stressed cells can be unable to maintain tolerance of autoreactive cells, and autoreactive cells can be less susceptible to the programmed cell death that occurs in cells with unrecoverable levels of ER stress.
-
The UPR represents a potential therapeutic target. Drugs that enhance UPR activity can be protective in autoimmune conditions such as ankylosing spondylitis, in which abnormal protein folding is thought to drive the disease, whereas drugs that impair the UPR may be beneficial in conditions in which malignant or autoreactive cells rely on the UPR for survival.
Abstract
Many exogenous sources of stress can lead to cell death. In recent years, endogenous cellular sources of stress have also been identified, including the stress that arises from the accumulation of unfolded proteins within a cell's endoplasmic reticulum (ER). To counterbalance this type of ER stress, higher eukaryotic cells possess a three-pronged signal-transduction pathway termed the unfolded-protein response (UPR). This Review focuses on the role of the UPR in the mammalian immune system and how manipulation of this complex signalling pathway may be of therapeutic benefit in human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Patil, C. & Walter, P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13, 349–355 (2001).
Cox, J. S. & Walter, P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404 (1996).
Travers, K. J. et al. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258 (2000).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Shi, Y. et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Biol. Cell 18, 7499–7509 (1998).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Lee, K. et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16, 452–466 (2002).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Bertolotti, A., Zhang, Y., Hendershot, L. M., Harding, H. P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biol. 2, 326–332 (2000).
Shen, J., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Kimata, Y. et al. Genetic evidence for a role of BiP/Kar2 that regulates Ire1 in response to accumulation of unfolded proteins. Mol. Biol. Cell 14, 2559–2569 (2003).
Credle, J. J., Finer-Moore, J. S., Papa, F. R., Stroud, R. M. & Walter, P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 18773–18784 (2005).
Kimata, Y. et al. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J. Cell Biol. 179, 75–86 (2007).
Wang, X. Z. et al. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 17, 5708–5717 (1998).
Bertolotti, A. et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1 β-deficient mice. J. Clin. Invest. 107, 585–593 (2001).
Tirasophon, W., Lee, K., Callaghan, B., Welihinda, A. & Kaufman, R. J. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 14, 2725–2736 (2000).
Shamu, C. E. & Walter, P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 15, 3028–3039 (1996).
Urano, F. & Wang, X. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase. Science 287, 664–666 (2000).
Nishitoh, H. et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355 (2002).
Ogata, M. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 26, 9220–9231 (2006).
Maiuri, M. C., Zalckvar, E., Kimchi, A. & Kroemer, G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature Rev. Mol. Cell Biol. 8, 741–752 (2007).
Yoneda, T. et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 276, 13935–13940 (2001).
Lee, A. H., Iwakoshi, N. N., Anderson, K. C. & Glimcher, L. H. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl Acad. Sci. USA 100, 9946–9951 (2003).
Tirosh, B., Iwakoshi, N. N., Glimcher, L. H. & Ploegh, H. L. Rapid turnover of unspliced Xbp-1 as a factor that modulates the unfolded protein response. J. Biol. Chem. 281, 5852–5860 (2006).
Yoshida, H., Oku, M., Suzuki, M. & Mori, K. pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J. Cell Biol. 172, 565–575 (2006).
Lee, A. H., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23, 7448–7459 (2003).
Liou, H. C. et al. A new member of the leucine zipper class of proteins that binds to the HLA DR α promoter. Science 247, 1581–1584 (1990).
Clauss, I. M., Chu, M., Zhao, J. L. & Glimcher, L. H. The basic domain/leucine zipper protein hXBP-1 preferentially binds to and transactivates CRE-like sequences containing an ACGT core. Nucleic Acids Res. 24, 1855–1864 (1996).
Clauss, I. M. et al. In situ hybridization studies suggest a role for the basic region-leucine zipper protein hXBP-1 in exocrine gland and skeletal development during mouse embryogenesis. Dev. Dyn. 197, 146–156 (1993).
Reimold, A. M. et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 14, 152–157 (2000).
Lee, A. H., Chu, G. C., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368–4380 (2005). This paper shows additional roles for XBP1 and the UPR in the genesis of secretory structures such as exocrine gland acinar cells.
Hetz, C. et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1α. Science 312, 572–576 (2006).
Gu, F. et al. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J. Biol. Chem. 279, 49689–49693 (2004).
Hetz, C. & Glimcher, L. The daily job of night killers: alternative roles of the BCL-2 family in organelle physiology. Trends Cell Biol. 18, 38–44 (2008).
Harding, H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000).
Lu, P. D., Harding, H. P. & Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27–33 (2004).
Vattem, K. M. & Wek, R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA 101, 11269–11274 (2004).
Novoa, I., Zeng, H., Harding, H. P. & Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 (2001).
Marciniak, S. J. et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004).
Chen, X., Shen, J. & Prywes, R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J. Biol. Chem. 277, 13045–13052 (2002).
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Bailey, D. & O'Hare, P. Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid. Redox Signal. 9, 2305–2321 (2007).
Brown, M. S. & Goldstein, J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 (1997).
Haze, K. et al. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem. J. 355, 19–28 (2001).
Thuerauf, D. J., Morrison, L. & Glembotski, C. C. Opposing roles for ATF6α and ATF6β in endoplasmic reticulum stress response gene induction. J. Biol. Chem. 279, 21078–21084 (2004).
Adachi, Y. et al. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Funct. 33, 75–89 (2008).
Yamamoto, K. et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 (2007).
Wu, J. et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 (2007).
Wang, Y. et al. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 275, 27013–27020 (2000).
Yoshida, H., Haze, K., Yanagi, H., Yura, T. & Mori, K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 273, 33741–33749 (1998).
Yoshida, H. et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20, 6755–6767 (2000).
Roy, B. & Lee, A. S. The mammalian endoplasmic reticulum stress response element consists of an evolutionarily conserved tripartite structure and interacts with a novel stress-inducible complex. Nucleic Acids Res. 27, 1437–1443 (1999).
Gass, J. N., Gunn, K. E., Sriburi, R. & Brewer, J. W. Stressed-out B cells? Plasma-cell differentiation and the unfolded protein response. Trends Immunol. 25, 17–24 (2004).
Shaffer, A. L. et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21, 81–93 (2004). This paper shows that the transcription factor BLIMP1 is upstream of XBP1 in plasma cells and is an important contribution to unmasking the network of transcription factors involved in plasma-cell development.
Shen, Y. & Hendershot, L. M. Identification of ERdj3 and OBF-1/BOB-1/OCA-B as direct targets of XBP-1 during plasma cell differentiation. J. Immunol. 179, 2969–2978 (2007).
Lee, A.-H., Scapa, E. F., Cohen, D. E. & Glimcher, L. H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320, 1492–1496 (2008).
Sriburi, R., Jackowski, S., Mori, K. & Brewer, J. W. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 167, 35–41 (2004).
Yan, W. et al. Control of PERK eIF2α kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc. Natl Acad. Sci. USA 99, 15920–15925 (2002).
van Huizen, R., Martindale, J. L., Gorospe, M. & Holbrook, N. J. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2α signaling. J. Biol. Chem. 278, 15558–15564 (2003).
Zinszner, H. et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 (1998).
Reimold, A. M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001). This is the first report showing that plasma-cell growth and survival are dependent on XBP1, although the UPR-dependent mechanism had yet to be identified.
Iwakoshi, N. N. et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nature Immunol. 4, 321–329 (2003).
Reimold, A. M. et al. Transcription factor B cell lineage-specific activator protein regulates the gene for human X-box binding protein 1. J. Exp. Med. 183, 393–401 (1996).
Chen, J., Lansford, R., Stewart, V., Young, F. & Alt, F. W. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc. Natl Acad. Sci. USA. 90, 4528–4532 (1993).
Gass, J. N., Gifford, N. M. & Brewer, J. W. Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J. Biol. Chem. 277, 49047–49054 (2002).
Tirosh, B., Iwakoshi, N. N., Glimcher, L. H. & Ploegh, H. L. XBP-1 specifically promotes IgM synthesis and secretion, but is dispensable for degradation of glycoproteins in primary B cells. J. Exp. Med. 202, 505–516 (2005).
Skalet, A. H. et al. Rapid B cell receptor-induced unfolded protein response in nonsecretory B Cells correlates with pro- versus antiapoptotic cell fate. J. Biol. Chem. 280, 39762–39771 (2005).
Rush, J. S., Sweitzer, T., Kent, C., Decker, G. L. & Waechter, C. J. Biogenesis of the endoplasmic reticulum in activated B lymphocytes: temporal relationships between the induction of protein N-glycosylation activity and the biosynthesis of membrane protein and phospholipid. Arch. Biochem. Biophys. 284, 63–70 (1991).
Wiest, D. L. et al. Membrane biogenesis during B cell differentiation: most endoplasmic reticulum proteins are expressed coordinately. J. Cell Biol. 110, 1501–1511 (1990).
Zhang, K. et al. The unfolded protein response sensor IRE1α is required at 2 distinct steps in B cell lymphopoiesis. J. Clin. Invest. 115, 268–281 (2005).
Gass, J. N., Jiang, H.-Y., Wek, R. C. & Brewer, J. W. The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells. Mol. Immunol. 45, 1035–1043 (2008).
Berland, R. & Wortis, H. H. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20, 253–300 (2002).
Genestier, L. et al. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J. Immunol. 178, 7779–7786 (2007).
Tumang, J. R., Frances, R., Yeo, S. G. & Rothstein, T. L. Cutting Edge: spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J. Immunol. 174, 3173–3177 (2005).
Shapiro-Shelef, M. et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19, 607–620 (2003).
Klein, U. et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nature Immunol. 7, 773–782 (2006).
Niu, H., Ye, B. H. & Dalla-Favera, R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 12, 1953–1961 (1998).
Turner, C. A., Mack, D. H. & Davis, M. M. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell 77, 297–306 (1994).
Lin, Y. & Wong, K.-K. Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science 276, 596–599 (1997).
Shaffer, A. L. et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Lin, K.-I., Angelin-Duclos, C., Kuo, T. C. & Calame, K. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol. Cell. Biol. 22, 4771–4780 (2002).
Rinkenberger, J. L., Wallin, J. J., Johnson, K. W. & Koshland, M. E. An interleukin-2 signal relieves BSAP (Pax5)-mediated repression of the immunoglobulin J chain gene. Immunity 5, 377–386 (1996).
Mittrucker, H.-W. et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science 275, 540–543 (1997).
Sciammas, R. et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 25, 225–236 (2006).
Iwakoshi, N. N., Pypaert, M. & Glimcher, L. H. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med. 204, 2267–2275 (2007). This paper shows that XBP1 is important for DC development and survival, which has implications for the role of the UPR in early facets of the immune response.
Omori, Y. et al. CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nucleic Acids Res. 29, 2154–2162 (2001).
Zhang, K. et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–599 (2006).
Ron, D. Proteotoxicity in the endoplasmic reticulum: lessons from the Akita diabetic mouse. J. Clin. Invest. 109, 443–445 (2002).
Yoshida, H. ER stress and diseases. FASEB J. 274, 630–658 (2007). A review that highlights the potential role of the UPR in various diseases, including neurodegenerative diseases and type 1 diabetes.
Schlosstein, L., Terasaki, P. I., Bluestone, R. & Pearson, C. M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N. Engl. J. Med. 288, 704–706 (1973).
Hammer, R. E., Maika, S. D., Richardson, J. A., Tang, J.-P. & Taurog, J. D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell 63, 1099–1112 (1990).
Mear, J. P. et al. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J. Immunol. 163, 6665–6670 (1999).
Dangoria, N. S. et al. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J. Biol. Chem. 277, 23459–23468 (2002).
Turner, M. J., Delay, M. L., Bai, S., Klenk, E. & Colbert, R. A. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: implications for the pathogenesis of spondylarthritis-like disease. Arthritis Rheum. 56, 215–223 (2007).
Malik, P., Klimovitsky, P., Deng, L.-W., Boyson, J. E. & Strominger, J. L. Uniquely conformed peptide-containing β2-microglobulin- free heavy chains of HLA-B2705 on the cell surface. J. Immunol. 169, 4379–4387 (2002).
Smith, J. A., Marker-Hermann, E. & Colbert, R. A. Pathogenesis of ankylosing spondylitis: current concepts. Best Pract. Res. Clin. Rheumatol. 20, 571–591 (2006). This is a review on the role of the UPR in the pathogenesis of ankylosing spondylitis based on research obtained from the HLA-B27 transgenic rat model of the disease.
Blais, A. et al. An initial blueprint for myogenic differentiation. Genes Dev. 19, 553–569 (2005).
Acosta-Alvear, D. et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 27, 53–66 (2007). A compelling study that uses genome-wide approaches to identify many putative XBP1 target genes, including Mist1.
Johnson, C. L., Kowalik, A. S., Rajakumar, N. & Pin, C. L. Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mech. Dev. 121, 261–272 (2004).
Vattemi, G., Engel, W. K., McFerrin, J. & Askanas, V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am. J. Pathol. 164, 1–7 (2004).
Nagaraju, K. et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 52, 1824–1835 (2005).
Nagaraju, K. et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc. Natl Acad. Sci. USA 97, 9209–9214 (2000).
Blass, S. et al. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 44, 761–771 (2001).
Corrigall, V. M. et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J. Immunol. 166, 1492–1498 (2001).
Blachere, N. E. et al. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic t lymphocyte response and tumor immunity. J. Exp. Med. 186, 1315–1322 (1997).
Purcell, A. W. et al. Association of stress proteins with autoantigens: a possible mechanism for triggering autoimmunity? Clin. Exp. Immunol. 132, 193–200 (2003).
Lin, W. et al. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J. Clin. Invest. 117, 448–456 (2007).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Li, Y. et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-α and interleukin-6: model of NF-κB- and MAP kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 280, 21763–21772 (2005).
Gargalovic, P. S. et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 2490–2496 (2006).
Bays, N. W., Gardner, R. G., Seelig, L. P., Joazeiro, C. A. & Hampton, R. Y. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nature Cell Biol. 3, 24–29 (2001).
Amano, T. et al. Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev. 17, 2436–2449 (2003).
Yamasaki, S., Yagishita, N., Tsuchimochi, K., Nishioka, K. & Nakajima, T. Rheumatoid arthritis as a hyper-endoplasmic reticulum-associated degradation disease. Arthritis Res. Ther. 7, 181–186 (2005).
Gao, B. et al. Synoviolin promotes IRE1 ubiquitination and degradation in synovial fibroblasts from mice with collagen-induced arthritis. EMBO Rep. 9, 480–485 (2008).
Tran, T. M. et al. Additional human β2-microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27-transgenic rats. Arthritis Rheum. 54, 1317–1327 (2006).
Carrasco, D. R. et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell 11, 349–360 (2007). This study connects myeloma-cell survival with XBP1 function and introduces a mouse model of multiple myeloma that is based on the overexpression of XBP1s.
Neubert, K. et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nature Med. 14, 748–755 (2008).
Marciniak, S. J. & Ron, D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 86, 1133–1149 (2006).
Katzel, J. A., Hari, P. & Vesole, D. H. Multiple myeloma: charging toward a bright future. CA Cancer J. Clin. 57, 301–318 (2007).
Kyle, R. A. & Rajkumar, S. V. Multiple myeloma. N. Engl. J. Med. 351, 1860–1873 (2004).
Obeng, E. A. et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107, 4907–4916 (2006).
Davenport, E. L. et al. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood 110, 2641–2649 (2007).
Acknowledgements
We would like to acknowledge F. Martinon, C. Hetz and P. Thielen for their critical review of the manuscript, and K. Nevis for preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
L.H.G. holds equity in, and is on the corporate board of directors of, the Bristol-Myers Squibb pharamceutical company.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Plasma cells
-
Non-dividing, terminally differentiated, immunoglobulinsecreting cells of the B-cell lineage.
- Autophagy
-
An evolutionarily conserved process in which acidic double-membrane vacuoles sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation through fusion to secondary lysosomes.
- Caspases
-
A family of cysteine proteinases that are involved in the initiation and effector stages of apoptosis.
- Ubiquitin
-
A small protein that is attached to other proteins by ubiquitin ligases. Depending on the mode of attachment, ubiquitin can either activate signalling function or target a protein for destruction by the proteasome.
- Programmed cell death
-
A common form of cell death that is also known as apoptosis. Many physiological and developmental stimuli cause apoptosis, and this mechanism is frequently used to delete unwanted, superfluous or potentially harmful cells, such as those undergoing transformation. Apoptosis involves cell shrinkage, chromatin condensation in the periphery of the nucleus, plasma-membrane blebbing and DNA fragmentation into segments of ∼180 base pairs. Eventually, the cell breaks up into many membrane-bound 'apoptotic bodies', which are phagocytosed by neighbouring cells.
- Recombination activating gene-2 (RAG2) blastocyst complementation system
-
A chimeric mouse model that is used to study the function of a gene in the adaptive immune system. This system is used when germline deletion of a gene of interest (for example, X-box binding protein 1 (XBP1)) results in an embryonic lethal phenotype. Embryonic stem cells from Xbp1−/− mice are injected into RAG-deficient blastocysts, with the resulting mouse being a chimera of the two. Because of the RAG mutation, mature B and T cells in these chimeric mice are strictly of the Xbp1−/− lineage.
- Class switching
-
The somatic-recombination process by which immunoglobulin isotypes are switched from IgM to IgG, IgA or IgE.
- Pro-B cell
-
A cell in the earliest stage of B-cell development in the bone marrow. Pro-B cells are characterized by incomplete immunoglobulin heavy-chain rearrangements and are defined as CD19+ cytoplasmic IgM− or, sometimes, as B220+CD43+ cells (by the Hardy classification scheme).
- B2 cells
-
Conventional B cells. B2 cells reside in secondary lymphoid organs and secrete antigen-specific antibodies.
- B1 cells
-
B1 cells express CD5, respond quickly to antigen, produce antibodies of broad specificity and do not depend on MHC class II-mediated T-cell help.
- Germinal centre
-
A lymphoid structure that arises in follicles after immunization with, or exposure to, a T-cell-dependent antigen. It is specialized for facilitating the development of high-affinity, long-lived plasma cells and memory B cells.
- Conventional DCs
-
A subset of dendritic cells (DCs) that already display the morphology and function of a DC under steady-state conditions.
- Plasmacytoid DCs
-
A subset of dendritic cells (DCs) that has a morphology which resembles that of a plasmablast. Plasmacytoid DCs produce large amounts of type I interferons in response to viral infection.
- Small interfering RNAs
-
(siRNAs). Short double-stranded RNAs of 19–23 nucleotides that induce RNA interference, a post-transcriptional process that leads to gene silencing in a sequence-specific manner.
- Immune tolerance
-
Denotes lymphocyte non-responsiveness to antigen, but implies an active process, not simply a passive lack of response.
- β2-microglobulin
-
(β2m). A single immunoglobulin-like domain that non-covalently associates with the main polypeptide chain of MHC class I molecules. In the absence of β2m, MHC class I molecules are unstable and are therefore found at very low levels at the cell surface.
- Epitope spreading
-
The de novo activation of autoreactive T cells by self-antigens that have been released after B- or T-cell-mediated bystander damage.
- Inflammatory bowel disease
-
(IBD). A group of conditions of unknown aetiology in which the intestinal mucosa is chronically inflamed. IBD includes Crohn's disease and ulcerative colitis.
- Experimental autoimmune encephalomyelitis
-
(EAE). An experimental model for the human disease multiple sclerosis. Autoimmune disease is induced in experimental animals by immunization with myelin or peptides derived from myelin. The animals develop a paralytic disease with inflammation and demyelination in the brain and spinal cord.
- Atherosclerosis
-
A chronic disorder of the arterial wall characterized by endothelial-cell damage that gradually induces deposits of cholesterol, cellular debris, calcium and other substances. These deposits finally lead to plaque formation and arterial stiffness.
- E3 ubiquitin ligase
-
The enzyme that is required to attach the molecular tag ubiquitin to proteins that are destined for degradation by the proteasomal complex.
Rights and permissions
About this article
Cite this article
Todd, D., Lee, AH. & Glimcher, L. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 8, 663–674 (2008). https://doi.org/10.1038/nri2359
Issue Date:
DOI: https://doi.org/10.1038/nri2359
This article is cited by
-
GBA1 as a risk gene for osteoporosis in the specific populations and its role in the development of Gaucher disease
Orphanet Journal of Rare Diseases (2024)
-
Control of immune cell function by the unfolded protein response
Nature Reviews Immunology (2023)
-
Negligible role of TRAIL death receptors in cell death upon endoplasmic reticulum stress in B-cell malignancies
Oncogenesis (2023)
-
FK506-binding protein 2 (FKBP13) inhibit Bax-induced apoptosis in Saccharomyces cerevisiae (yeast)
Cell Biology and Toxicology (2023)
-
High-fat diet alters intestinal microbiota and induces endoplasmic reticulum stress via the activation of apoptosis and inflammation in blunt snout bream
Fish Physiology and Biochemistry (2023)