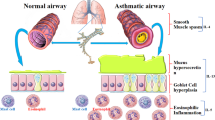
Key Points
-
Remarkable progress has been made in recent years on the structural determination of proteins in the IgE network and on the functions and regulation of IgE. This is beginning to feed into IgE-targeted therapies for allergy.
-
The shape of the IgE molecule differs dramatically from that of IgG. X-ray and nuclear magnetic resonance studies have also revealed conformational changes that occur on IgE binding to its high-affinity receptor FcεRI (high-affinity Fc receptor for IgE) on mast cells and antigen-presenting cells, events that lead, respectively, to sensitization (and the immediate hypersensitivity reaction) and the facilitation of allergen presentation.
-
The structural data also provide clues to the unique nature of the high-affinity IgE–FcεRI interaction, and indicate possibilities for blocking the interaction; validation of IgE as a target is demonstrated by the success of the IgE-specific monoclonal antibody omalizumab in the treatment of asthma.
-
The presence of an unusually long extracellular membrane-proximal domain in membrane IgE may also determine its ability to act as an antigen receptor on B cells and to respond to particular antigens (allergens).
-
The trimeric structure of the C-type lectin, low-affinity IgE receptor CD23, and its susceptibility to cleavage by ADAM10 (a disintegrin and metalloproteinase 10) at the cell surface, provide important clues to the mechanism of IgE homeostasis.
-
CD23 has multiple ligands, including IgE, CD21 and various integrins, enabling it to carry out several other functions, including IgE-dependent antigen presentation and cellular cytotoxicity.
-
IgE is transported to mucosal tissues by CD23 and is also synthesized by the resident B cells. Its concentration is maintained in the tissue by the number of mast cells that express FcεRI at high levels and the slow rate of dissociation of IgE from FcεRI.
-
Class switching to IgE and affinity maturation of the antibodies occur in mucosal tissues, and this may limit the ability of IgE antibodies to mediate systemic anaphylaxis.
-
Various mechanisms contrive to suppress the production of IgE synthesis to tolerable levels, as well as limiting its anatomical distribution. Some mechanisms operate at the level of class-switch recombination, others at the level of survival of the IgE-switched cells.
-
IgE transport in both directions through the gastrointestinal epithelium may be involved in early sensitization to allergens. Studies of the mechanism of early sensitization may suggest means of preventing the development of allergic disease.
-
Small molecule inhibitors of the IgE–FcεRI interaction may supersede IgE-specific antibodies, but the combination of this approach with immunotherapy may be required for more effective therapeutic intervention in allergy and asthma.
Abstract
The spreading epidemic of allergies and asthma has heightened interest in IgE, the central player in the allergic response. The activity of IgE is associated with a network of proteins; prominent among these are its two principal receptors, FcεRI (high-affinity Fc receptor for IgE) and CD23, as well as galectin-3 and several co-receptors for CD23, notably CD21 and various integrins. Here, we review recent progress in uncovering the structures of these proteins and their complexes, and in our understanding of how IgE exerts its effects and how its expression is regulated. The information that has emerged suggests new therapeutic directions for combating allergic disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Stanworth, D. R. The discovery of IgE. Allergy 48, 67–71 (1993).
Thornton, C. A. et al. Fetal exposure to intact immunoglobulin E occurs via the gastrointestinal tract. Clin. Exp. Allergy 33, 306–311 (2003).
Finkelman, F. D. & Vercelli, D. Advances in asthma, allergy mechanisms, and genetics in 2006. J. Allergy Clin. Immunol. 120, 544–550 (2007).
Gould, H. J. et al. The biology of IgE and the basis of allergic disease. Annu. Rev. Immunol. 21, 579–628 (2003).
Holgate, S. et al. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J. Allergy Clin. Immunol. 115, 459–465 (2005).
Zheng, Y., Shopes, B., Holowka, D. & Baird, B. Conformations of IgE bound to its receptor FcεRI and in solution. Biochemistry 30, 9125–9132 (1991). This paper provides the first demonstration of the bent structure of IgE both in solution and bound to the cell.
Zheng, Y., Shopes, B., Holowka, D. & Baird, B. Dynamic conformations compared for IgE and IgG1 in solution and bound to receptors. Biochemistry 31, 7446–7456 (1992).
Wan, T. et al. The crystal structure of IgE Fc reveals an asymmetrically bent conformation. Nature Immunol. 3, 681–686 (2002). This X-ray study provides a high-resolution structure for the bent IgE-Fc and shows the location of the Cε2 domains for the first time.
Beavil, A. J., Young, R. J., Sutton, B. J. & Perkins, S. J. Bent domain structure of recombinant human IgE-Fc in solution by X-ray and neutron scattering in conjunction with an automated curve fitting procedure. Biochemistry 34, 14449–14461 (1995).
Wurzburg, B. A., Garman, S. C. & Jardetzky, T. S. Structure of the human Cε3-Cε4 reveals conformational flexibility in the antibody effector domains. Immunity 13, 375–385 (2000).
Bestagno, M. et al. Membrane immunoglobulins are stabilised by interchain disulphide bonds occurring within the extracellular membrane-proximal domain. Biochemistry 40, 10686–10692 (2001).
Batista, F. D., Anand, S., Presani, G., Efremov, D. G. & Burrone, O. R. The two membrane isoforms of human IgE assemble into functionally distinct B cell antigen receptors. J. Exp. Med. 184, 2197–2205 (1996). This study demonstrates that the two isoforms of membrane IgE ('long' and 'short'), as part of the BCR, transmit different signals.
Vangelista, L. et al. Membrane IgE binds and activates FcεRI in an antigen-independent manner. J. Immunol. 174, 5602–5611 (2005).
Kinet, J.-P. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu. Rev. Immunol. 17, 931–972 (1999).
Kraft, S. & Kinet, J.-P. New developments in FcεRI regulation, function and inhibition. Nature Rev. Immunol. 7, 365–378 (2007).
Garman, S. C., Kinet, J.-P. & Jardetzky, T. S. Crystal structure of the human high-affinity IgE receptor. Cell 95, 951–961 (1998).
Garman, S. C., Sechi, S., Kinet, J.-P. & Jardetzky, T. S. The analysis of the human high affinity IgE receptor FcεRIα from multiple crystal forms. J. Mol. Biol. 311, 1049–1062 (2001).
Garman, S. C., Wurzburg, B. A., Tarchevskaya, S. S., Kinet, J.-P. & Jardetzky, T. S. Structure of the Fc fragment of human IgE bound to its high-affinity receptor FcεRIα. Nature 406, 259–266 (2000). This paper reports the X-ray crystal structure of the complex formed between an IgE-Fc fragment consisting of the Cε3 and Cε4 domains, and the extracellular region of FcεRI α-chain.
Henry, A. J. et al. Participation of the N-terminal region of Cε3 in the binding of human IgE to its high-affinity receptor FcεRI. Biochemistry 36, 15568–15578 (1997).
McDonnell, J. M. et al. The structure of the IgE Cε2 domain and its role in stabilizing the complex with its high-affinity receptor FcεRI. Nature Struct. Biol. 8, 437–441 (2001).
Holowka, D., Sil, D., Torigoe, C. & Baird, B. Insights into immunoglobulin E receptor signalling from structurally defined ligands. Immunol. Rev. 217, 269–279 (2007).
Niemi, M. et al. Molecular interactions between a recombinant IgE antibody and the β-lactoglobulin allergen. Structure 15, 1413–1421 (2007).
Weskamp, G. et al. ADAM 10 is a principal “sheddase” of the low-affinity immunoglobulin E receptor CD23. Nature Immunol. 7, 1293–1298 (2006).
Lemieux, G. A. et al. The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J. Biol. Chem. 282, 14836–14844 (2007).
McCloskey, N. et al. Soluble CD23 monomers inhibit and oligomers stimulate IgE synthesis in human B cells. J. Biol. Chem. 282, 24083–24091 (2007).
Kijimoto-Ochiai, S. & Noguchi, A. Two peptides from CD23, including the inverse RGD sequence and its related peptide, interact with the MHC class II molecule. Biochem. Biophys. Res. Commun. 267, 686–691 (2000).
Hibbert, R. G. et al. The structure of human CD23 and its interactions with IgE and CD21. J. Exp. Med. 202, 751–760 (2005). This NMR study reports the solution structure of the lectin head domain of CD23, and maps the binding sites for IgE and CD21.
Yokota, A. et al. Two forms of the low-affinity Fc receptor for IgE differentially mediate endocytosis and phagocytosis: identification of the critical cytoplasmic domains. Proc. Natl Acad. Sci. USA 89, 5030–5034 (1992).
Montagnac G. et al. Intracellular trafficking of CD23: differential regulation in humans and mice by both extracellular and intracellular exons. J. Immunol. 174, 5562–5572 (2005).
Vercelli, D. et al. The B-cell binding site on human immunoglobulin E. Nature 338, 649–651 (1989).
Aubry, J.-P., Pochon, S., Graber, P., Jansen, K. U. & Bonnefoy, J.-Y. CD21 is a ligand for CD23 and regulates IgE production. Nature 358, 505–507 (1992).
Aubry, J.-P. et al. CD23 interacts with a new functional extracytoplasmic domain involving N-linked oligosaccharides on CD21. J. Immunol. 152, 5806–5813 (1994).
Wurzburg, B. A., Tarchevskaya S. S. & Jardetzky, T. S. Structural changes in the lectin domain of CD23, the low-affinity IgE receptor, upon calcium binding. Structure 14, 1049–1058 (2006).
Bettler, B. et al. Immunoglobin E-binding site in Fcε receptor (FcεRII/CD23) identified by homolog-scanning mutagenesis. J. Biol. Chem. 267, 185–191 (1992).
Mossalayi, M. et al. Cytokine effects of CD23 are mediated by an epitope distinct from the IgE binding site. EMBO J. 11, 4323–4328 (1992).
Szakonyi, G. et al. Structure of complement receptor 2 in complex with its C3d ligand. Science 292, 1725–1728 (2001).
Gilbert, H. E., Asokan, R., Holers, V. M. & Perkins, S. J. The 15 SCR flexible extracellular domains of human complement receptor type 2 can mediate multiple ligand and antigen interactions. J. Mol. Biol. 362, 1132–1147 (2006).
Sayers, I., Housden, J. E. M., Spivey, A. C. & Helm, B. A. The importance of Lys-352 of human immunoglobulin E in FcεRII/CD23 recognition. J. Biol. Chem. 279, 35320–35325 (2004).
Liu, F.-T. Regulatory roles of galectins in the immune response. Int. Arch. Allergy Immunol. 136, 385–400 (2005).
Ahmad, N. et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279, 10841–10847 (2004).
Seetharaman, J. et al. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1Å resolution. J. Biol. Chem. 273, 13047–13052 (1998).
Lecoanet-Henchoz, S. et al. CD23 regulates monocyte activation through a novel interaction with the adhesion molecules CD11b–CD18 and CD11c–CD18. Immunity 3, 119–125 (1995).
Lecoanet-Henchoz, S. et al. Mouse CD23 regulates monocyte activation through an interaction with the adhesion molecule CD11b/CD18. Eur. J. Immunol. 27, 2290–2294 (1997).
Hermann, P. et al. The vitronectin receptor and its associated CD47 molecule mediates proinflammatory cytokine synthesis in human monocytes by interaction with soluble CD23. J. Cell Biol. 144, 767–775 (1999).
Borland, G. et al. αvβ5 integrin sustains growth of human pre-B cells through an RGD-independent interaction with a basic domain of the CD23 protein. J. Biol. Chem. 282, 27315–27326 (2007).
Kaur. D., Berger, P., Duffy, D. E. Brightling, C. E. & Bradding, P. Co-cultivation of mast cells and FcεRIα+ dendritic-like cells from human hip bone marrow. Clin. Exp. Allergy 35, 226–233 (2005).
MacGlashan, D. Jr. IgE and FcεRI regulation. Ann. NY Acad. Sci. 1050, 73–88 (2005).
Smurthwaite, L. et al. Persistent IgE synthesis in the nasal mucosa of hayfever patients. Eur. J. Immunol. 31, 3422–3431 (2001). This study demonstrates that IgE synthesis keeps the mast cells permanently sensitized to allergens in the nasal mucosa of allergic rhinitis patients.
Wilson, D. R. et al. Increases in allergen-specific IgE in BAL after segmental allergen challenge in atopic asthmatics. Am. J. Resp. Crit. Care Med. 165, 22–26 (2002).
Negrao-Correa, D., Adams, L. S. & Bell, R. G. Intestinal transport and catabolism of IgE: a major blood-independent pathway of IgE dissemination during Trichenella spiralis infection of rats. J. Immunol. 157, 4037–4046 (1996).
Kawakami, T. & Galli, S. J. Regulation of mast cell and basophil function and survival by IgE. Nature Rev. Immunol. 2, 773–786 (2002).
KleinJan, A., Vinke, J. G., Severinjnen, L. W. F. M. & Fokkens, W. J. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur. Respir. J. 15, 491–497 (2000). This paper shows that the abundance of IgE-expressing B cells and plasma cells in the nasal mucosa of allergic rhinitis patients is several orders of magnitude higher than in the circulation.
Hibi, T. & Dosch, H. M. Limiting dilution analysis of the B cell compartment in human bone marrow. Eur. J. Immunol. 16, 139–145 (1986).
Snow, R. E., Djukanovic, R. & Stevenson, F. K. Analysis of immunoglobulin E VH transcripts in a bronchial biopsy of an asthmatic patient confirms bias towards VH5 and indicates local clonal expansion, somatic mutation and isotype switch events. Immunology 98, 646–651 (1999).
Coker, H. A., Durham, S. R. & Gould, H. J. Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients. J. Immunol. 171, 5602–5610 (2003).
Cameron, L. et al. SεSμ and SεSγ switch circles in human nasal mucosa following ex vivo allergen challenge: evidence for direct as well as sequential class switch recombination. J. Immunol. 171, 3816–3822 (2003).
Takhar, P. et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J. Immunol. 174, 5024–5032 (2005). This study demonstrates that class switching to IgE occurs locally in the target organ of allergic disease.
Gould, H. J. et al. Germinal-centre reactions in allergic inflammation. Trends Immunol. 27, 446–452 (2006).
Takhar, P. et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 119, 213–219 (2007).
Radbruch, A. et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nature Rev. Immunol. 6, 741–750 (2006).
Hiepe, F. & Radbruch, A. Is long-term humoral immunity in the mucosa provided by long-lived plasma cells? A question still open. Eur. J. Immunol. 36, 1068–1069 (2006).
Fagarasan, S. & Honjo, T. Regulation of IgA synthesis at mucosal surfaces. Curr. Opin. Immunol. 16, 277–283 (2004).
Fagarasan, S. et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298, 1424–1427 (2002).
Weiner, H. L. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol. Today 18, 335–343 (1997).
Bieber, T. The pro- and anti-inflammatory properties of human antigen-presenting cells expressing the high affinity receptor for IgE (FcεRI). Immunobiology 212, 499–503 (2007).
Coeffier, M., Lorenz, A., Manis, M. P. & Bischoff, S. C. ε germline and IL-4 transcripts are expressed human intestinal mucosa and enhanced in patients with food allergy. Allergy 60, 822–827 (2005).
Sutton, B. J. & Gould, H. J. The human IgE network. Nature 366, 421–428 (1993).
Carlsson, F., Hjelm, F., Conrad, D. H. & Heyman, B. IgE enhances specific antibody and T cell responses in mice over-expressing CD23. Scand. J. Immunol. 66, 261–270 (2007).
Karagiannis, S. N. et al. Endocytosis and recycling of the complex between CD23 and HLA-DR in human B cells. Immunology 103, 319–331 (2001).
Mudde, G. C., Bheekha, R. & Bruijnzeel-Koomen, C. A. Consequences of IgE/CD23-mediated antigen presentation in allergy. Immunol. Today 16, 380–383 (1995).
Lewis, G. et al. Hyper IgE in New Zealand black mice due to a dominant-negative CD23 mutation. Immunogenetics 56, 564–571 (2004).
Kaminski, D. A. & Stavnezer, J. Antibody class switching differs among SJL, C57BL and 129 mice. Int. Immunol. 19, 545–556 (2007).
Meng, J. F., McFall, C. & Rosenwasser, L. J. Polymorphism R62W results in resistance of CD23 to enzymatic cleavage in cultured cells. Genes Immun. 8, 215–223 (2007).
Dombrowicz, D. & Capron, M. Eosinophils, allergy and parasites. Curr. Opin. Immunol. 13, 716–720 (2001).
Karagiannis, S. N. et al. IgE antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J. Immunol. 179, 2832–2843 (2007).
Plater-Zyberk, C. & Bonnefoy, J.-Y. Marked amelioration of established collagen-induced arthritis by treatment with antibodies to CD23 in vivo. Nature Med. 1, 781–785 (1995).
Zuberi, R. I. et al. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am. J. Pathobiol. 165, 2045–2053 (2004).
Chen, H.-Y. et al. Role of galectin-3 in mast cell functions: galectin-3-deficient mast cells exhibit impaired mediator release and defective JNK expression. J. Immunol. 177, 4991–4997 (2006).
Kaiserlian, D. et al. Intestinal epithelial cells express the CD23/FcεRII molecule: enhanced expression in enteropathies. Immunology 80, 90–95 (1993).
Tu, Y. et al. CD23-mediated IgE transport across human intestinal epithelium: inhibition by blocking sites of translation or binding. Gastroenterology 129, 928–940 (2005). This study demonstrates that membrane CD23 transports IgE from the gastrointestinal mucosa across the epithelial-cell barrier into the lumen, and transports allergen–IgE complexes in the opposite direction.
Berin, M. C., Li, H. & Sperber, K. Antibody-mediated antigen sampling across intestinal epithelial barriers. Ann. NY Acad. Sci. 1072, 253–261 (2006).
Yu, L. C. & Perdue, M. H. Role of mast cells in intestinal mucosal function: studies in models of hypersensitivity and stress. Immunol. Rev. 179, 61–73 (2001).
Lima, J. O. et al. Early expression of Iε, CD23 (FcεRII) IL-4-α and IgE in the human fetus. J. Allergy Clin. Immunol. 106, 911–917 (2000).
Bergmann, R. L. et al. Predictability of early atopy by cord blood IgE and parental history. Clin. Exp. Allergy 27, 752–760 (1997).
Conrad, D. H., Ford, J. W., Sturgill, B. S. & Gibb, D. R. CD23: an overlooked regulator of allergic disease. Curr. Allergy Asthma Rep. 7, 331–337 (2007).
Gould, H. J. et al. IgE homeostasis: is CD23 the safety switch? In IgE regulation: molecular mechanisms (ed. Vercelli, D) 37–59 (J. Wiley & Sons Ltd, Chichester, UK, 1997).
Fearon, D. T. & Carroll, M. C. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol. 18, 393–422 (2000).
Yu, P. et al. Negative feedback regulation of IgE synthesis by murine CD23. Nature 369, 753–756 (1994).
Rosenwasser, L. J. & Meng, J. Anti-CD23. Clin. Rev. Allergy Immunol. 29, 61–72 (2005).
Tangye, S. G., Avery, D. T. & Hodgkin, P. D. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory cells. J. Immunol. 170, 261–269 (2003).
Yan, C. T. et al. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature 449, 478–482 (2007).
Fear, D. J., McCloskey, N. M., O'Connor, B. O., Felsenfeld, G. & Gould, H. J. Transcription of Ig germline genes in single human B cells and the role of cytokines in isotype determination. J. Immunol. 173, 4529–4538 (2004).
Dudley, D. D. et al. Internal IgH class switch region deletions are position-independent and enhanced by AID expression. Proc. Natl Acad. Sci. USA 99, 9984–9989 (2002).
Karnowski, A., Achatz-Straussberger, G., Klockenbusch, C., Achatz, G. & Lamers, M. C. Inefficient processing of mRNA for the membrane form of IgE in a genetic mechanism to limit recruitment of IgE-secreting cells. Eur. J. Immunol. 36, 1917–1925 (2006). This study shows that the RNA chain termination signals at the 3′ end of the gene encoding the ε-chain of IgE differ from all other isotypes, and explains why IgE expression is less efficient than other isotypes.
Karnowski, A., Yu, P., Achatz, G. & Lamers, M. C. The road to the production of IgE is long and winding. Am. J. Respir. Crit. Care Med. 162, S71–S75 (2000).
Chang, T. W. The pharmacological basis of anti-IgE therapy. Nature Biotechnol. 18, 157–162 (2000).
Stamos, J. et al. Convergent recognition of the IgE binding site on the high-affinity IgE receptor. Structure 12, 1289–1301 (2004).
Ober, C. & Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 7, 95–100 (2006).
Vercelli, D. Discovering susceptibility genes for asthma and allergy. Nature Rev. Immunol. 8, 169–182 (2008).
Lau, S. et al. Longitudinal study on the relationship between cat allergen and endotoxin exposure, sensitisation, cat-specific IgG and development of asthma in childhood. Allergy 60, 766–773 (2005).
Maleki, S. J., Chung, S. Y., Champagne, E. T. & Raufman, J. P. The effects of roasting on the allergenic properties of peanut proteins. J. Allergy Clin. Immunol. 107, 763–768 (2000).
Grimshaw, K. E. C. et al. Presentation of allergen in different food preparations affects the nature of the allergic reaction — a case series. Clin. Exp. Allergy 33, 1581–1585 (2002).
Untersmayr, R. et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 19, 656–658 (2005).
Diaz-Sanchez, D., Dotson, A. R., Takenaka, H. & Saxon, A. Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms. J. Clin. Invest. 94, 1417–1425 (1994).
Kusel, M. M. H. et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 119, 1105–1110 (2007).
Pérez-Yarza, E. G., Moreno, A., Lázaro, P., Mejías, A. & Ramilo, O. The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatr. Infec. Dis. J. 26, 733–739 (2007).
Gould, H. J., Takhar, P., Harries, H. E., Chevretton, E. & Sutton, B. J. The allergic march from Staphylococcus aureus superantigens to immunoglobulin E. Chem. Immunol. Allergy 93, 106–136 (2007).
Bachert, C., Gevaert, P., Zhang, N., van Zele, T. & Perez-Novo, C. Role of staphylococcal superantigens in airway disease. Chem. Immunol. Allergy 93, 214–236 (2007).
Garn, H., Mittermann, I., Valenta, R. & Renz, H. Autosensitization as a pathomechanism in asthma. Ann. NY Acad. Sci. 1107, 417–425 (2007).
Mothes, N. et al. The cradle of IgE autoreactivity in atopic eczema lies in early infancy. J. Allergy Clin. Immunol. 116, 706–709 (2005).
Acknowledgements
We acknowledge the support of the Medical Research Council (UK), The Wellcome Trust and Asthma UK for our work in this field. We also thank K. Kirwan, A. Davies and R. Beavil for help with preparation of the original figures.
Author information
Authors and Affiliations
Glossary
- Fluorescence resonance energy transfer
-
(FRET). A quantum mechanical process by which excitation energy is transferred, without the emission of a photon, from a donor fluorophore to an acceptor fluorophore that is in close proximity. FRET can be used to determine inter- or intra-molecular distances (in the range of 10–100 Å).
- Extracellular membrane-proximal domain
-
(EMPD). A domain present in each heavy chain of a membrane immunoglobulin, located between the C-terminal domain of the soluble antibody (Cε4 in IgE) and the transmembrane sequence.
- Antigen-presenting cells
-
(APCs). Cells that can internalize and process antigen, and then display antigenic peptide fragments on their surface, together with molecules required to activate the cognate lymphocytes.
- Immunoreceptor tyrosine-based activation motif
-
(ITAM). A short peptide motif containing tyrosine residues that is found in the cytoplasmic tail of several signalling adaptor proteins and that is necessary to recruit proteins that are involved in triggering activating signalling proteins. The consensus sequence is Tyr-X-X-(Leu/Ile)-X6–8-Tyr-X-X-(Leu/Ile), where X denotes any amino acid.
- C-type (calcium dependent) lectin superfamily
-
A family of calcium-dependent carbohydrate-binding proteins. The binding activity of C-type lectins is based on the structure of the carbohydrate-recognition domain (CRD), which is highly conserved across this family. Calcium contributes to the structural maintenance of this domain and typically is essential for its carbohydrate-binding function.
- Follicular dendritic cell
-
(FDC). A cell type that is normally found only in the germinal centres of lymphoid tissue and that presents antigen to selected B cells and provides survival signals required for affinity maturation.
- RGD sequence
-
A peptide motif that consists of the amino acids arginine, glycine and aspartic acid, common to many ligands that bind integrins.
- Early phase of the allergic reaction
-
The biological and clinical consequences that occur within the first hour of crosslinking complexes of IgE with FcεRI (high-affinity Fc receptor for IgE) at the surface of mast cells and/or basophils by allergens. The clinical manifestations are characterized by tissue-specific effects, including constriction of the large airways in asthma, and 'wheal-and-flare' reactions in the skin. Generalized symptoms in multiple target organs can include oedema and pruritus (itching). Systemic manifestations can include angio-oedema, urticaria and, in severe cases, vascular collapse (anaphylaxis).
- Late phase of the allergic reaction
-
Clinical manifestations can be measurable (visible) two or more hours after allergen exposure, but may also appear much later. These manifestations peak at 6–9 hours after allergen exposure and will have resolved by 24–48 hours. Reactions are characterized by oedema and the infiltration of T helper 2 cells and eosinophils. Tissue reactions are characterized by oedema, pain, warmth and erythema (redness). Reactions in the lungs are characterized by airway narrowing and mucus hypersecretion.
- Class-switch recombination
-
(CSR). The process by which a heavy-chain variable region gene segment attached to one heavy-chain constant region gene segment in the expressed heavy-chain gene is recombined with a downstream constant region gene segment to express a new antibody class.
- Somatic hypermutation
-
(SHM). The process by which point mutations occur in the heavy- or light-chain variable region gene segments, resulting in a change in the expressed protein, which may alter the antigen (or allergen) affinity or specificity.
- Affinity maturation
-
The process by which B cells are selected for survival and proliferation on the basis of their affinity for antigen.
- Langerhans cells
-
Professional antigen-presenting dendritic cells localized in the skin epidermis.
- Facilitated antigen presentation
-
(FAP). Also known as facilitated allergen presentation, this is the process by which CD23 internalizes allergen–IgE complexes and recycles peptides complexed with MHC class II molecules to the cell surface for T-cell recognition.
- Epitope spreading
-
The process by which an antibody response to one epitope on an antigen leads to the production of antibodies specific for other epitopes on the same antigen, or for epitopes on entirely unrelated antigens. This results from the internalization of whole antigen and subsequent display of a range of peptides derived from that antigen, leading to the generation of T cells with different epitope specificities. Simultaneous processing of two unrelated antigens by an antigen-presenting cell can lead to the production of antibodies directed against both antigens.
- Atopy
-
A condition, mediated by IgE antibodies, of increased susceptibility to immediate hypersensitivity.
Rights and permissions
About this article
Cite this article
Gould, H., Sutton, B. IgE in allergy and asthma today. Nat Rev Immunol 8, 205–217 (2008). https://doi.org/10.1038/nri2273
Issue Date:
DOI: https://doi.org/10.1038/nri2273
This article is cited by
-
The role of regulatory B cells in immune regulation and childhood allergic asthma
Molecular and Cellular Pediatrics (2024)
-
Dose–Response Effect of Saccharomyces cerevisiae UFMG A-905 on the Prevention of Asthma in an Animal Model
Probiotics and Antimicrobial Proteins (2024)
-
Carriers of the p.P522R variant in PLCγ2 have a slightly more responsive immune system
Molecular Neurodegeneration (2023)
-
Molecular engineering of nanobodies as tools in allergology: diagnostics and beyond
Allergo Journal International (2023)
-
Molekulares Design von Nanobodies als Werkzeuge in der Allergologie: Diagnostik und mehr
Allergo Journal (2023)