Key Points
-
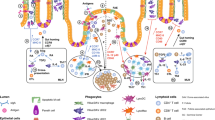
An important feature of the intestinal immune system is its ability to protect against infection while avoiding the development of destructive inflammatory responses to the normal microbiota and dietary antigens.
-
Populations of intestinal dendritic cells (DCs) display unique functional properties. They have been implicated both in the maintenance of tolerance towards harmless antigens, and in the generation of protective immune responses against pathogens.
-
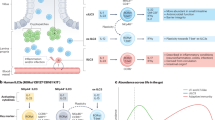
Recent data suggests that intestinal DCs can promote differentiation of forkhead box P3 (FOXP3)+ regulatory T cells from naive precursors. Transforming growth factor-β and retinoic acid have an important role in this process.
-
Conditioning of DCs in their local tissue environment may have an important role in shaping their function.
-
A breakdown in intestinal homeostasis can result in chronic inflammatory diseases of the gut including inflammatory bowel disease. Inappropriate or aberrant DC function may have a role in the pathogenesis of inflammatory bowel disease.
Abstract
A breakdown in intestinal homeostasis can result in chronic inflammatory diseases of the gut including inflammatory bowel disease, coeliac disease and allergy. Dendritic cells, through their ability to orchestrate protective immunity and immune tolerance in the host, have a key role in shaping the intestinal immune response. The mechanisms through which dendritic cells can respond to environmental cues in the intestine and select appropriate immune responses have until recently been poorly understood. Here, we review recent work that is beginning to identify factors responsible for intestinal conditioning of dendritic-cell function and the subsequent decision between tolerance and immunity in the intestine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Iwasaki, A. Mucosal dendritic cells. Annu. Rev. Immunol. 25, 381–418 (2007).
Smith, P. D., Ochsenbauer-Jambor, C. & Smythies, L. E. Intestinal macrophages: unique effector cells of the innate immune system. Immunol. Rev. 206, 149–159 (2005).
Johansson, C. & Kelsall, B. L. Phenotype and function of intestinal dendritic cells. Semin. Immunol. 17, 284–294 (2005).
Iwasaki, A. & Kelsall, B. L. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190, 229–239 (1999).
Chirdo, F. G., Millington, O. R., Beacock-Sharp, H. & Mowat, A. M. Immunomodulatory dendritic cells in intestinal lamina propria. Eur. J. Immunol. 35, 1831–1840 (2005).
Iwasaki, A. & Kelsall, B. L. Unique functions of CD11b+, CD8α+, and double-negative Peyer's patch dendritic cells. J. Immunol. 166, 4884–4890 (2001).
Annacker, O., et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 202, 1051–1061 (2005).
Iwasaki, A. & Kelsall, B. L. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191, 1381–1394 (2000).
Smythies, L. E., et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115, 66–75 (2005).
Denning, T. L., Wang, Y. C., Patel, S. R., Williams, I. R. & Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature Immunol. 8, 1086–1094 (2007).
Naik, S. H., et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nature Immunol. 7, 663–671 (2006).
Wilson, N. S., et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood 102, 2187–2194 (2003).
Fogg, D. K., et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311, 83–87 (2006).
Varol, C., et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 204, 171–180 (2007).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Yrlid, U., Jenkins, C. D. & MacPherson, G. G. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J. Immunol. 176, 4155–4162 (2006).
Yoshida, M., et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20, 769–783 (2004).
Jang, M. H., et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl Acad. Sci. USA 101, 6110–6115 (2004).
Rescigno, M., et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2, 361–367 (2001).
Chieppa, M., Rescigno, M., Huang, A. Y. & Germain, R. N. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 203, 2841–2852 (2006).
Niess, J. H., et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Turnbull, E. L., Yrlid, U., Jenkins, C. D. & Macpherson, G. G. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J. Immunol. 174, 1374–1384 (2005).
Wilson, N. S., et al. Normal proportion and expression of maturation markers in migratory dendritic cells in the absence of germs or Toll-like receptor signaling. Immunol. Cell Biol. 86, 200–205 (2008).
Macpherson, A. J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
Huang, F. P., et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000).
Worbs, T., et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203, 519–527 (2006).
Jiang, A., et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity 27, 610–624 (2007). This study provided details of a distinctive programme of DC maturation initiated by disruption of E-cadherin-mediated DC–DC interactions.
Wendland, M., et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc. Natl Acad. Sci. USA 104, 6347–6352 (2007).
Yrlid, U., et al. Regulation of intestinal dendritic cell migration and activation by plasmacytoid dendritic cells, TNF-α and type 1 IFNs after feeding a TLR7/8 ligand. J. Immunol. 176, 5205–5212 (2006).
Sun, C. M., et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Mucida, D., et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Yamazaki, S., et al. Dendritic cells are specialized accessory cells along with TGF-β for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3− precursors. Blood 110, 4293–4302 (2007).
Coombes, J. L., et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Kang, S. G., Lim, H. W., Andrisani, O. M., Broxmeyer, H. E. & Kim, C. H. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J. Immunol. 179, 3724–3733 (2007). These papers, together with reference 10, demonstrated an important role for populations of MLN DCs, lamina propria DCs and lamina propria macrophages in the generation of FOXP3+ T Reg cells from naive peripheral CD4+ T cells. This was found to be mediated in part by retinoic acid, which enhanced TGFβ-mediated induction of FOXP3.
Johansson-Lindbom, B., et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 (2005).
Travis, M. A., et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361–365 (2007). This study provided physiological evidence that DCs, through the activation of TGFβ, can influence intestinal immune homeostasis.
Lacy-Hulbert, A., et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc. Natl Acad. Sci. USA 104, 15823–15828 (2007).
Fahlen, L., et al. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J. Exp. Med. 201, 737–746 (2005).
Bilsborough, J., George, T. C., Norment, A. & Viney, J. L. Mucosal CD8α+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology 108, 481–492 (2003).
Stagg, A. J., Kamm, M. A. & Knight, S. C. Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur. J. Immunol. 32, 1445–1454 (2002).
Johansson-Lindbom, B., et al. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198, 963–969 (2003).
Mora, J. R., et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Briskin, M., et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 151, 97–110 (1997).
Kunkel, E. J., et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 (2000).
Siewert, C., et al. Induction of organ-selective CD4+ regulatory T cell homing. Eur. J. Immunol. 37, 978–989 (2007).
Macpherson, A. J. & Slack, E. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr. Opin. Gastroenterol. 23, 673–678 (2007).
Martinoli, C., Chiavelli, A. & Rescigno, M. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity 27, 975–984 (2007).
Sato, A., et al. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J. Immunol. 171, 3684–3690 (2003).
He, B., et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 (2007).
Cerutti, A. The regulation of IgA class switching. Nature Rev. Immunol. 8, 421–434 (2008).
Benson, M. J., Pino-Lagos, K., Rosemblatt, M. & Noelle, R. J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204, 1765–1774 (2007).
Schambach, F., Schupp, M., Lazar, M. A. & Reiner, S. L. Activation of retinoic acid receptor-α favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur. J. Immunol. 37, 2396–2399 (2007).
Iwata, M., et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Mora, J. R., et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160 (2006). This study showed that gut-associated lymphoid tissue DCs could promote expression of gut-homing receptors by B cells. This was mediated by retinoic acid. Retinoic acid also synergized with IL-5 and IL-6 to promote secretion of IgA.
McDermott, M. R., et al. Impaired intestinal localization of mesenteric lymphoblasts associated with vitamin A deficiency and protein-calorie malnutrition. Immunology 45, 1–5 (1982).
Stephensen, C. B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 21, 167–192 (2001).
Elias, K. M., et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111, 1013–1020 (2008).
Blomhoff, R. & Blomhoff, H. K. Overview of retinoid metabolism and function. J. Neurobiol. 66, 606–630 (2006).
Saurer, L., McCullough, K. C. & Summerfield, A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J. Immunol. 179, 3504–3514 (2007).
Lampen, A., Meyer, S., Arnhold, T. & Nau, H. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J. Pharmacol. Exp. Ther. 295, 979–985 (2000).
Svensson, M. e. a. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 1, 38–48 (2008).
Cantorna, M. T., Nashold, F. E. & Hayes, C. E. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J. Immunol. 152, 1515–1522 (1994).
Sidell, N., Chang, B. & Bhatti, L. Upregulation by retinoic acid of interleukin-2-receptor mRNA in human T lymphocytes. Cell. Immunol. 146, 28–37 (1993).
Rincon, M. & Flavell, R. A. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 13, 4370–4381 (1994).
Wu, Y., et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 126, 375–387 (2006).
Schule, R., et al. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc. Natl Acad. Sci. USA 88, 6092–6096 (1991).
Fantini, M. C., et al. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172, 5149–5153 (2004).
Artis, D. Epithelial-cell recognition of commensal flora and maintenance of immune homeostasis in the gut. Nature Rev. Immunol. 8, 411–421 (2008).
Rimoldi, M., et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nature Immunol. 6, 507–514 (2005). This study emphasised the importance of interactions between epithelial cells and DCs in the regulation of intestinal immune responses. DCs cultured with epithelial-cell supernatants were 'conditioned' to produce less IL-12 and promote T H 2-type responses. This effect was in part mediated by TSLP.
Zaph, C., et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007). This paper deals with the crosstalk between the intestinal epithelium and DCs. Mice with an IEC-specific deletion of IKKβ have impaired expression of TSLP, which results in elevated production of IL-12/23p40 by DCs and failure to mount protective T H 2-type responses following Trichuris muris infection.
Watanabe, N., et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436, 1181–1185 (2005).
Szatmari, I., et al. PPARγ controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 203, 2351–2362 (2006).
Kobayashi, M., et al. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Invest. 111, 1297–1308 (2003).
Takeda, K., et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49 (1999).
Butler, M., et al. Modulation of dendritic cell phenotype and function in an in vitro model of the intestinal epithelium. Eur. J. Immunol. 36, 864–874 (2006).
Rakoff-Nahoum, S., Hao, L. & Medzhitov, R. Role of Toll-like receptors in spontaneous commensal-dependent colitis. Immunity 25, 319–329 (2006).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Contractor, N., Louten, J., Kim, L., Biron, C. A. & Kelsall, B. L. Cutting edge: Peyer's patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFβ, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J. Immunol. 179, 2690–2694 (2007).
Rimoldi, M., et al. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood 106, 2818–2826 (2005).
Sierro, F., et al. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl Acad. Sci. USA 98, 13722–13727 (2001).
Nenci, A., et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 (2007).
Becker, C., et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Invest. 112, 693–706 (2003).
Salazar-Gonzalez, R. M., et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity 24, 623–632 (2006). This study revealed that T-cell activation following Salmonella spp . infection was dependent on the recruitment of CCR6-expressing DCs to the follicle-associated epithelium of the Peyer's patches.
Bettelli, E., et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Hart, A. L., et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology 129, 50–65 (2005).
Uhlig, H. H., et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Berndt, B. E., Zhang, M., Chen, G. H., Huffnagle, G. B. & Kao, J. Y. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J. Immunol. 179, 6255–6262 (2007).
Abe, K., et al. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc. Natl Acad. Sci. USA 104, 17022–17027 (2007).
Watanabe, N., et al. Elimination of local macrophages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig Dis. Sci. 48, 408–414 (2003).
Strober, W., Murray, P. J., Kitani, A. & Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nature Rev. Immunol. 6, 9–20 (2006).
Inohara, N., et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278, 5509–5512 (2003).
Li, J., et al. Regulation of IL-8 and IL-1β expression in Crohn's disease associated NOD2/CARD15 mutations Hum. Mol. Genet. 13, 1715–1725 (2004).
Uehara, A., et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell. Microbiol. 7, 53–61 (2005).
Netea, M. G., et al. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J. Immunol. 174, 6518–6523 (2005).
van Heel, D. A., et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn's disease. Lancet 365, 1794–1796 (2005).
van Beelen, A. J., et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27, 660–669 (2007).
Happel, K. I., et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202, 761–769 (2005).
Ivanov, I. I., et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Mangan, P. R., et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Kobayashi, K. S., et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005).
Watanabe, T., et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J. Clin. Invest. 118, 545–559 (2008).
Watanabe, T., Kitani, A., Murray, P. J. & Strober, W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nature Immunol. 5, 800–808 (2004).
Maeda, S., et al. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1β processing. Science 307, 734–738 (2005).
Duerr, R. H., et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Yen, D., et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116, 1310–1316 (2006).
Hue, S., et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203, 2473–2483 (2006).
Kullberg, M. C., et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 203, 2485–2494 (2006).
Izcue, A., et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 28, 559–570 (2008).
Sugimoto, K., et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Jang, M. H., et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J. Immunol. 176, 803–810 (2006).
Karlis, J., et al. Characterization of colonic and mesenteric lymph node dendritic cell subpopulations in a murine adoptive transfer model of inflammatory bowel disease. Inflamm. Bowel Dis. 10, 834–847 (2004).
Liu, L. M. & MacPherson, G. G. Rat intestinal dendritic cells: immunostimulatory potency and phenotypic characterization. Immunology 85, 88–93 (1995).
Kilshaw, P. J. Expression of the mucosal T cell integrin αM290 β7 by a major subpopulation of dendritic cells in mice. Eur. J. Immunol. 23, 3365–3368 (1993).
Brenan, M. & Puklavec, M. The MRC OX-62 antigen: a useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J. Exp. Med. 175, 1457–1465 (1992).
Asselin-Paturel, C., Brizard, G., Pin, J. J., Briere, F. & Trinchieri, G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 171, 6466–6477 (2003).
Yrlid, U., et al. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J. Immunol. 177, 6115–6121 (2006).
Balmer, J. E. & Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 43, 1773–1808 (2002).
La, P., et al. Fusion proteins of retinoid receptors antagonize TGF-b-induced growth inhibition of lung epithelial cells. Oncogene 22, 198–210 (2003).
Pendaries, V., Verrecchia, F., Michel, S. & Mauviel, A. Retinoic acid receptors interfere with the TGF-b/Smad signaling pathway in a ligand-specific manner. Oncogene 22, 8212–8220 (2003)
Acknowledgements
The authors are supported by the Wellcome trust (F.P.), the European Union (Euro-Thymaide FP6 Integrated Project; LSHB-CT-2003-503410), and the Medical Research Council (J.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Inflammatory bowel disease
-
(IBD). A group of conditions, of unknown aetiology, in which the intestinal mucosa is chronically inflammed. Includes Crohn's disease and ulcerative colitis.
- Plasmacytoid DC
-
(pDCs). A subset of dendritic cells (DCs) the microscopic appearance of which resembles plasmablasts. In humans, these DCs can be derived from lineage-negative stem cells in peripheral blood and are the main producers of type I interferons (IFNs) in response to virus infections. Recent studies have identified subsets of type-I-IFN-producing DCs in mice, which are identified by expression of B220, Ly6C and other markers.
- M cells
-
(Microfold cells). Specialized epithelial cells that deliver antigens by transepithelial vesicular transport from the gut lumen directly to intraepithelial lymphocytes and to subepithelial lymphoid tissues.
- Regulatory T cell
-
(TReg cell). A type of CD4+ T cell that is characterized by its expression of forkhead box P3 (FOXP3) and high levels of CD25. TReg cells can downmodulate many types of immune responses. CD4+CD25+FOXP3+ TReg cells that develop in the thymus are exported to the periphery with their regulatory function already intact. However, FOXP3+ T cells with regulatory function can also be generated in the periphery from naive CD4+ T cells following, for example, the oral administration of antigen or targeting of peptide ligands to dendritic cells in vivo.
- TH17 cells
-
(T helper 17 cells). A subset of CD4+ T helper cells that produce interleukin-17 (IL-17) and that are thought to be important in inflammatory and autoimmune diseases. Their generation involves IL-6, TGFβ (transforming growth factor-β), IL-23 and IL-21, as well as the transcription factors RORγt (retinoic-acid-receptor-related orphan receptor-γt) and STAT3 (signal transducer and activator of transcription 3).
Rights and permissions
About this article
Cite this article
Coombes, J., Powrie, F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol 8, 435–446 (2008). https://doi.org/10.1038/nri2335
Issue Date:
DOI: https://doi.org/10.1038/nri2335
This article is cited by
-
Role of Rho GTPases in inflammatory bowel disease
Cell Death Discovery (2023)
-
Gut microbiota regulates chronic ethanol exposure-induced depressive-like behavior through hippocampal NLRP3-mediated neuroinflammation
Molecular Psychiatry (2023)
-
An updated review on oral protein-based antigen vaccines efficiency and delivery approaches: a special attention to infectious diseases
Archives of Microbiology (2023)
-
Mechanistic Insights into Immune-Microbiota Interactions and Preventive Role of Probiotics Against Autoimmune Diabetes Mellitus
Probiotics and Antimicrobial Proteins (2023)
-
Microbiome diversity declines while distinct expansions of Th17, iNKT, and dendritic cell subpopulations emerge after anastomosis surgery
Gut Pathogens (2021)