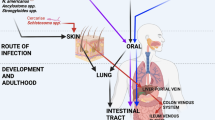
Key Points
-
Helminth parasites cause chronic disease in billions of people, however, the immune response associated with helmith infection can also reduce the severity of certain harmful inflammatory autoimmune and allergic diseases.
-
Studies of tissue-dwelling parasites in murine models reveal the development of T helper 2 (TH2)-type granulomas consisting of cellular infiltrates that resemble TH1-type granulomas; however, the cells are activated differently and have distinct functions.
-
The TH2-type response can affect host protection by mediating helminth expulsion or by controlling otherwise pathological inflammatory responses that are driven by TH1 and TH17 cells.
-
TH2 cells are the primary source of TH2-type cytokines but innate cells can also produce these cytokines.
-
TH2-type cytokines, including interleukin-4 (IL-4) and IL-13, orchestrate a potent TH2-type response by direct stimulation of both bone-marrow-derived and non-bone-marrow-derived cell populations.
-
A similarly complex and multi-faceted TH2-type response is elicited following infection with a wide variety of helminths; however, only certain components of this broad response are effective against a particular species.
-
The discovery of new effector cell types and molecules contributing to the host protective TH2-type response provides additional targets for the development of novel therapies against helminths.
Abstract
Important insights have recently been gained in our understanding of how host immune responses mediate resistance to parasitic helminths and control associated pathological responses. Although similar cells and cytokines are evoked in response to infection by helminths as diverse as nematodes and schistosomes, the components of the response that mediate protection are dependent on the particular parasite. In this Review, we examine recent findings regarding the mechanisms of protection in helminth infections that have been elucidated in murine models and discuss the implications of these findings in terms of future therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
WHO in World Health Report (World Health Organization, Geneva, Switzerland, 1999).
Capron, A., Riveau, G., Capron, M. & Trottein, F. Schistosomes: the road from host-parasite interactions to vaccines in clinical trials. Trends Parasitol. 21, 143–149 (2005).
Goud, G. N. et al. Expression of the Necator americanus hookworm larval antigen Na-ASP-2 in Pichia pastoris and purification of the recombinant protein for use in human clinical trials. Vaccine 23, 4754–4764 (2005).
Fallon, P. G. & Mangan, N. E. Suppression of TH2-type allergic reactions by helminth infection. Nature Rev. Immunol. 7, 220–230 (2007).
Wilson, M. S. & Maizels, R. M. Regulation of allergy and autoimmunity in helminth infection. Clin. Rev. Allergy Immunol. 26, 35–50 (2004).
Strachan, D. P. Hay fever, hygiene, and household size. BMJ 299, 1259–1260 (1989).
Holt, P. G. Parasites, atopy, and the hygiene hypothesis: resolution of a paradox? Lancet 356, 1699–1701 (2000).
Yazdanbakhsh, M. & Matricardi, P. M. Parasites and the hygiene hypothesis: regulating the immune system? Clin. Rev. Allergy Immunol. 26, 15–24 (2004).
Yazdanbakhsh, M., Kremsner, P. G. & van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 296, 490–494 (2002). A review of the inverse relationship of helminth infections and atopic diseases.
Owyang, A. M. et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203, 843–849 (2006).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Jankovic, D. et al. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204, 273–283 (2007).
Anderson, C. F., Oukka, M., Kuchroo, V. J. & Sacks, D. CD4+CD25−Foxp3− Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204, 285–297 (2007).
Hoffman, K. F., Cheever, A. W. & Wynn, T. A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine response induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164, 6406–6416 (2000).
Gause, W. C., Urban, J. F. Jr & Stadecker, M. J. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 24, 269–277 (2003).
Stadecker, M. J. et al. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol. Rev. 201, 168–179 (2004).
Wynn, T. A. Fibrotic disease and the TH1/TH2 paradigm. Nature Rev. Immunol. 4, 583–594 (2004).
Anthony, R. M. et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature Med. 12, 955–960 (2006). This study identifies alternatively activated macrophages as antihelminthic effector cells.
Morimoto, M. et al. Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J. Immunol. 172, 2424–2430 (2004).
Cliffe, L. J. & Grencis, R. K. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 57, 255–307 (2004).
Liu, Z. et al. Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunol. Rev. 201, 57–74 (2004).
Doenhoff, M. J. Granulomatous inflammation and the transmission of infection: schistosomiasis—and TB too? Immunol. Today 19, 462–467 (1998).
Kreider, T., Anthony, R. M., Urban, J. F. Jr. & Gause, W. C. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 19, 448–453 (2007).
Mantovani, A., Sica, A. & Locati, M. Macrophage polarization comes of age. Immunity 23, 344–346 (2005).
Rodriguez-Sosa, M. et al. Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2-biasing ability. Infect. Immun. 70, 3656–3664 (2002).
Herbert, D. R. et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 (2004). This paper describes the regulatory potential of alternatively activated macrophages in the context of helminth infection.
Gupta, R. et al. Macrophages in the development of protective immunity against experimental Brugia malayi infection. Parasitology 129, 311–323 (2004).
Taylor, M. D., Harris, A., Nair, M. G., Maizels, R. M. & Allen, J. E. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J. Immunol. 176, 6918–6927 (2006).
Reece, J. J., Siracusa, M. C. & Scott, A. L. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect. Immun. 74, 4970–4981 (2006).
Anderson, C. F. & Mosser, D. M. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J. Leukoc. Biol. 72, 101–106 (2002).
Mosser, D. M. The many faces of macrophage activation. J. Leukoc. Biol. 73, 209–212 (2003).
Pesce, J. et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J. Clin. Invest. 116, 2044–2055 (2006).
Hesse, M. et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533–6544 (2001). This study characterizes the iNOS/arginase-1 balance in classically activated and alternatively activated macrophages in the context of infectious disease.
Flores Villanueva, P. O., Harris, T. S., Ricklan, D. E. & Stadecker, M. J. Macrophages from schistosomal egg granulomas induce unresponsiveness in specific cloned Th-1 lymphocytes in vitro and down-regulate schistosomal granulomatous disease in vivo. J. Immunol. 152, 1847–1855 (1994).
Atochina, O., Daly-Engel, T., Piskorska, D., McGuire, E. & Harn, D. A. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1+ macrophages that suppress naive CD4+ T cell proliferation via an IFN-γ and nitric oxide-dependent mechanism. J. Immunol. 167, 4293–4302 (2001).
Martin, P. & Leibovich, S. J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599–607 (2005).
Sakthianandeswaren, A. et al. The wound repair response controls outcome to cutaneous leishmaniasis. Proc. Natl Acad. Sci. USA 102, 15551–15556 (2005).
Gratchev, A. et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein βIG-H3. Scand. J. Immunol. 53, 386–392 (2001).
Gratchev, A., Schledzewski, K., Guillot, P. & Goerdt, S. Alternatively activated antigen-presenting cells: molecular repertoire, immune regulation, and healing. Skin Pharmacol. Appl. Skin Physiol. 14, 272–279 (2001).
Nair, M. G., Guild, K. J. & Artis, D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J. Immunol. 177, 1393–1399 (2006). This is a comprehensive review of the ChaFF family of molecules during T H 2-type responses.
Tsuda, Y. et al. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 21, 215–226 (2004).
Galioto, A. M. et al. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect. Immun. 74, 5730–5738 (2006).
Broide, D. H. Molecular and cellular mechanisms of allergic disease. J. Allergy Clin. Immunol. 108, S65–S71 (2001).
Ganley-Leal, L. M. et al. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect. Immun. 74, 2169–2176 (2006).
Brunet, L. R., Sabin, E. A., Cheever, A. W., Kopf, M. A. & Pearce, E. J. Interleukin 5 (IL-5) is not required for expression of a Th2 response or host resistance mechanisms during murine schistosomiasis mansoni but does play a role in development of IL-4-producing non-T, non-B cells. Infect. Immun. 67, 3014–3018 (1999).
Herndon, F. J. & Kayes, S. G. Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J. Immunol. 149, 3642–3647 (1992).
Saeftel, M., Arndt, M., Specht, S., Volkmann, L. & Hoerauf, A. Synergism of γ interferon and interleukin-5 in the control of murine filariasis. Infect. Immun. 71, 6978–6985 (2003).
Swartz, J. M. et al. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood 108, 2420–2427 (2006).
Knott, M. L. et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int. J. Parasitol. 37, 1367–1378 (2007).
Voehringer, D., Reese, T. A., Huang, X., Shinkai, K. & Locksley, R. M. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J. Exp. Med. 203, 1435–1446 (2006).
Ramalingam, T., Porte, P., Lee, J. & Rajan, T. V. Eosinophils, but not eosinophil peroxidase or major basic protein, are important for host protection in experimental Brugia pahangi infection. Infect. Immun. 73, 8442–8443 (2005).
Sabin, E. A., Kopf, M. A. & Pearce, E. J. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J. Exp. Med. 184, 1871–1878 (1996).
Reiman, R. M. et al. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect. Immun. 74, 1471–1479 (2006).
Padigel, U. M., Lee, J. J., Nolan, T. J., Schad, G. A. & Abraham, D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect. Immun. 74, 3232–3238 (2006).
Holland, M. J., Harcus, Y. M., Balic, A. & Maizels, R. M. Th2 induction by Nippostrongylus secreted antigens in mice deficient in B cells, eosinophils or MHC class I-related receptors. Immunol. Lett. 96, 93–101 (2005).
Lee, J. J. & Lee, N. A. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin. Exp. Allergy 35, 986–994 (2005).
Min, B. et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J. Exp. Med. 200, 507–517 (2004).
Shinkai, K., Mohrs, M. & Locksley, R. M. Helper T cells regulate type-2 innate immunity in vivo. Nature 420, 825–829 (2002).
Arinobu, Y. et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc. Natl Acad. Sci. USA 102, 18105–18110 (2005).
Behnke, J. M., Lowe, A., Clifford, S. & Wakelin, D. Cellular and serological responses in resistant and susceptible mice exposed to repeated infection with Heligmosomoides polygyrus bakeri. Parasite Immunol. 25, 333–340 (2003).
Gerken, S. E., Vaz, N. M. & Mota-Santos, T. A. Local anaphylactic reactions to the penetration of cercariae of Schistosoma mansoni. Braz. J. Med. Biol. Res. 23, 275–281 (1990).
Grencis, R. K., Else, K. J., Huntley, J. F. & Nishikawa, S. I. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 15, 55–59 (1993).
Newlands, G. F., Miller, H. R., MacKellar, A. & Galli, S. J. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N. brasiliensis infection. Blood 86, 1968–1976 (1995).
Knight, P. A., Wright, S. H., Lawrence, C. E., Paterson, Y. Y. & Miller, H. R. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 192, 1849–1856 (2000).
McDermott, J. R. et al. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl Acad. Sci. USA 100, 7761–7766 (2003).
Pennock, J. L. & Grencis, R. K. The mast cell and gut nematodes: damage and defence. Chem. Immunol. Allergy 90, 128–140 (2006).
Finkelman, F. D. et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 201, 139–155 (2004).
Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nature Immunol. 6, 507–514 (2005).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007). This study provides the identification of the epithelial-cell inflammatory pathway as being essential to induce a polarized T H 2-type response to helminth infection.
Cliffe, L. J. et al. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308, 1463–1465 (2005). This paper describes a role for epithelial-cell turnover in the protective response to helminth infection.
Hsieh, G. C. et al. A secreted protein from the human hookworm Necator americanus binds selectively to NK cells and induces IFN-γ production. J. Immunol. 173, 2699–2704 (2004).
Tliba, O., Chauvin, A., Le Vern, Y., Boulard, C. & Sbille, P. Evaluation of the hepatic NK cell response during the early phase of Fasciola hepatica infection in rats. Vet. Res. 33, 327–332 (2002).
McDermott, J. R., Humphreys, N. E., Forman, S. P., Donaldson, D. D. & Grencis, R. K. Intraepithelial NK cell-derived IL-13 induces intestinal pathology associated with nematode infection. J. Immunol. 175, 3207–3213 (2005).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nature Immunol. 5, 987–995 (2004).
Liu, Z. et al. IL-2 and autocrine IL-4 drive the in vivo development of antigen-specific Th2 T cells elicited by nematode parasites. J. Immunol. 174, 2242–2249 (2005).
Holland, M. J., Harcus, Y. M., Riches, P. L. & Maizels, R. M. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur. J. Immunol. 30, 1977–1987 (2000).
Okano, M., Satoskar, A. R., Nishizaki, K., Abe, M. & Harn, D. A. Jr Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J. Immunol. 163, 6712–6717 (1999).
Hokke, C. H. & Yazdanbakhsh, M. Schistosome glycans and innate immunity. Parasite Immunol. 27, 257–264 (2005).
Akdis, C. A. et al. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur. J. Immunol. 33, 2717–2726 (2003).
van der Kleij, D. et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277, 48122–48129 (2002).
Phillips, C., Coward, W. R., Pritchard, D. I. & Hewitt, C. R. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J. Leukoc. Biol. 73, 165–171 (2003).
Reese, T. A. et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447, 92–96 (2007). This study provides the identification of chitin as a PAMP recognized by T H 2-type responses.
Jankovic, D., Kullberg, M. C., Caspar, P. & Sher, A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J. Immunol. 173, 2419–2427 (2004).
Cervi, L., MacDonald, A. S., Kane, C., Dzierszinski, F. & Pearce, E. J. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J. Immunol. 172, 2016–2020 (2004).
Madden, K. B. et al. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J. Immunol. 172, 5616–5621 (2004).
Shea-Donohue, T. et al. The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J. Immunol. 167, 2234–2239 (2001).
Madden, K. B. et al. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J. Immunol. 169, 4417–4422 (2002).
Pearce, E. J. & MacDonald, A. S. The immunobiology of schistosomiasis. Nature Rev. Immunol. 2, 499–511 (2002).
Davies, S. J. et al. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science 294, 1358–1361 (2001).
Rutitzky, L. I., Lopes da Rosa, J. R. & Stadecker, M. J. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J. Immunol. 175, 3920–3926 (2005). This study describes the role of T H 17 cells in severe schistosomiasis.
Cua, D. J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003). This study identifies IL-23, instead of IL-12, as being responsible for driving harmful pro-inflammatory responses.
Aggarwal, S., Ghilardi, N., Xie, M. H., de Sauvage, F. J. & Gurney, A. L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 (2003).
Rutitzky, L. I., Hernandez, H. J. & Stadecker, M. J. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc. Natl Acad. Sci. USA 98, 13243–13248 (2001).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Saunders, K. A., Raine, T., Cooke, A. & Lawrence, C. E. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 75, 397–407 (2007).
McInnes, I. B. et al. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J. Immunol. 171, 2127–2133 (2003).
van den Biggelaar, A. H. et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet 356, 1723–1727 (2000).
Summers, R. W. et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 98, 2034–2041 (2003).
Belkaid, Y. & Rouse, B. T. Natural regulatory T cells in infectious disease. Nature Immunol. 6, 353–360 (2005).
Taylor, M. D. et al. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 174, 4924–4933 (2005).
Metwali, A. et al. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G253–G259 (2006).
Hesse, M. et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 172, 3157–3166 (2004).
Freeman, C. M. et al. CCR8 is expressed by antigen-elicited, IL-10-producing CD4+CD25+ T cells, which regulate Th2-mediated granuloma formation in mice. J. Immunol. 174, 1962–1970 (2005).
Baumgart, M., Tompkins, F., Leng, J. & Hesse, M. Naturally occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J. Immunol. 176, 5374–5387 (2006).
Taylor, J. J., Mohrs, M. & Pearce, E. J. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J. Immunol. 176, 5839–5847 (2006).
McKee, A. S. & Pearce, E. J. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J. Immunol. 173, 1224–1231 (2004).
Boyce, J. A. Mast cells: beyond IgE. J. Allergy Clin. Immunol. 111, 24–32 (2003).
Dawicki, W. & Marshall, J. S. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 19, 31–38 (2007).
Rajan, B., Ramalingam, T. & Rajan, T. V. Critical role for IgM in host protection in experimental filarial infection. J. Immunol. 175, 1827–1833 (2005).
Shibuya, A. et al. Fcα/μ receptor mediates endocytosis of IgM-coated microbes. Nature Immunol. 1, 441–446 (2000).
van Remoortere, A. et al. Dominant antibody responses to Fucα1–3GalNAc and Fucα1-2Fucα1-3GlcNAc containing carbohydrate epitopes in Pan troglodytes vaccinated and infected with Schistosoma mansoni. Exp. Parasitol. 105, 219–225 (2003).
Liu, Q. et al. The role of B cells in the development of CD4 effector T cells during a polarized Th2 immune response. J. Immunol. 179, 3821–3830 (2007).
Hernandez, H. J., Wang, Y. & Stadecker, M. J. In infection with Schistosoma mansoni, B cells are required for T helper type 2 cell responses but not for granuloma formation. J. Immunol. 158, 4832–4837 (1997).
Jankovic, D. et al. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J. Exp. Med. 187, 619–629 (1998).
Hagan, P., Blumenthal, U. J., Dunn, D., Simpson, A. J. & Wilkins, H. A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349, 243–245 (1991).
Harris, N. L. et al. Mechanisms of neonatal mucosal antibody protection. J. Immunol. 177, 6256–6262 (2006).
Hotez, P. J. et al. New technologies for the control of human hookworm infection. Trends Parasitol. 22, 327–331 (2006).
Nair, M. G. et al. Chitinase and fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect. Immun. 73, 385–394 (2005).
Steppan, C. M. et al. The hormone resistin links obesity to diabetes. Nature 409, 307–312 (2001).
Zhu, Z. et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304, 1678–1682 (2004). This study describes a role for alternatively activated macrophages in T H 2-type inflammation, albeit a harmful one in allergic models.
Sandler, N. G., Mentink-Kane, M. M., Cheever, A. W. & Wynn, T. A. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J. Immunol. 171, 3655–3667 (2003).
Loke, P. et al. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 3, 7 (2002).
Guo, L., Johnson, R. S. & Schuh, J. C. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J. Biol. Chem. 275, 8032–8037 (2000).
Welch, J. S. et al. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J. Biol. Chem. 277, 42821–42829 (2002).
Artis, D. et al. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl Acad. Sci. USA 101, 13596–13600 (2004).
Hogan, S. P. et al. Resistin-like molecule β regulates innate colonic function: barrier integrity and inflammation susceptibility. J. Allergy Clin. Immunol. 118, 257–268 (2006).
Schinke, T. et al. Cloning and functional characterization of resistin-like molecule gamma. Biochem. Biophys. Res. Commun. 314, 356–362 (2004).
Wang, M. L. et al. Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1074–G1083 (2005).
Chomarat, P. & Banchereau, J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int. Rev. Immunol. 17, 1–52 (1998).
Mentink-Kane, M. M. et al. IL-13 receptor α2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc. Natl Acad. Sci. USA 101, 586–590 (2004).
Morimoto, M. et al. Functional importance of regional differences in localized gene expression of receptors for IL-13 in murine gut. J. Immunol. 176, 491–495 (2006).
Zhao, A. et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 171, 948–954 (2003).
Falcone, F. H. et al. A Brugia malayi homolog of macrophage migration inhibitor factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J. Immunol. 167, 5348–5354 (2001).
Chang, N. C. et al. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J. Biol. Chem. 276, 17497–17506 (2001).
Acknowledgements
We greatly appreciate the careful review and thoughtful suggestions provided by F.D. Finkelman and E.J. Pearce, and would like to thank T. Kreider for his help in editing the original manuscript and drawing the original figures. This work was supported by National Institutes of Health Grants AI031678 and AI066188.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Helminths
-
These are worms that are characterized by their body shape into cestodes (flat worms), nematodes (round worms) and trematodes (leaf-shaped worms).
- Hygiene hypothesis
-
This hypothesis originally proposed that the increased incidence of atopic diseases in westernized countries was a consequence of living in an overly clean environment resulting in an under-stimulated immune system that responded inappropriately to harmless antigens. More recently it has been proposed that an absence of exposure to pathogens, in particular helminths, may predispose to both increased allergy and autoimmune disease.
- TH2-type response
-
(T-helper-2-type response).An immune response including innate and adaptive components that is elicited by helminth infections and many allergic reactions. Common features include expression of TH2-type cytokines (IL-4, IL-5 and IL-13), eosinophilia, basophilia, mastocytosis, goblet-cell hyperplasia and IgE production.
- TH1 cells
-
(T helper 1 cells). This is a CD4+ effector T cell mainly characterized by its expression of interferon-γ.
- Heligmosomoides polygyrus
-
A natural mouse gastro-intestinal trichostrongylid nematode parasite, used as a model of human intestinal nematode infection. Primary infections become established and chronic, and can be cleared by helminth-specific drug treatment. Challenge infections are naturally cleared by the host by day 14 post-infection, making this an excellent model of protective memory T-helper-2-type responses.
- Schistosoma mansoni
-
A blood-dwelling trematode parasite that causes a major form of human hepato-intestinal schistosomiasis. Infection of mice with this parasite represents a well-established murine model of this disease.
- Granuloma
-
A circumscribed inflammatory cell infiltrate surrounding a nidus. Its composition usually includes T cells and macrophages, and in the case of helminth infection, eosinophils.
- TH17 cell
-
(T helper 17 cell). This is a CD4+ effector T cell that expresses interleukin-17 (IL-17), IL-6, tumour necrosis factor, IL-21 and IL-22, but does not express interferon-γ nor IL-4.
- Alternatively activated macrophage
-
A macrophage stimulated by interleukin-4 (IL-4) or IL-13 that expresses arginase-1, mannose receptor CD206 and IL-4 receptor α. There may be pathogen-associated molecular patterns expressed by helminths that can also drive alternative activation of macrophages.
- Classically activated macrophage
-
A macrophage that is activated through Toll-like receptors and interferon-γ that expresses inducible nitric oxide synthase and nitric oxide.
- Filarial parasites
-
These thread-like nematode parasites cause a wide range of diseases in humans, including River blindness (by Onchocerca volvulus) and elephantiasis (by Wuchereria bancrofti, Brugia malayi and Brugia timori).
- Chitinase and FIZZ family member proteins
-
(ChaFFs). Proteins that are expressed by alternatively activated macrophages or goblet cells during TH2-type responses. They include acidic mammalian chitinase (AMCase), Ym1, Ym2, resistin-like molecule (RELMα also known as FIZZ1), RELMβ (also known as FIZZ 2) and RELMγ.
- Strongyloides stercoralis
-
A parasitic roundworm (threadworm) of humans and other mammals.
- Goblet cells
-
Mucus-producing cells found in the epithelial-cell lining of the intestine and lungs.
- Allergic cascade
-
The sequence of events contributing to acute and chronic allergic reactions. Surface Fc receptors for IgE on mast cells are crosslinked, triggering the release of soluble mediators. Immediate vascular permeability and smooth muscle contractility is associated with acute allergic reactions, and eosinophils and T helper 2 cells are associated with chronic allergic reactions.
- Nippostrongylus brasiliensis
-
A trichostrongylid intestinal nematode rodent parasite that is widely used as a model to study T-helper-2-type responses. In most inbred mouse strains, primary infection results in rapid expulsion of the parasite within 10–12 days.
- Toll-like receptors
-
(TLRs). The best characterized family of receptors for pathogen-associated molecular patterns. Receptors in this family recognize bacterial cell-wall and membrane structures, flagella, bacterial DNA motifs, viral double-stranded RNA and other structures.
- Pathogen-associated molecular patterns
-
(PAMPs). Conserved microbial structures recognized by innate receptors, including Toll-like receptors.
- Regulatory T cell
-
(TReg cell). A type of CD4+ T cell that is characterized by its expression of forkhead box P3 (FOXP3) and high levels of CD25. TReg cells can downmodulate many types of immune responses.
- Class switching
-
The somatic-recombination process by which the class of immunoglobulin is switched from IgM to IgG, IgA or IgE. During T helper 1 (TH1)-type responses, B cells can class-switch to produce IgG2a whereas during TH2-type responses B cells can switch to produce IgE.
Rights and permissions
About this article
Cite this article
Anthony, R., Rutitzky, L., Urban, J. et al. Protective immune mechanisms in helminth infection. Nat Rev Immunol 7, 975–987 (2007). https://doi.org/10.1038/nri2199
Issue Date:
DOI: https://doi.org/10.1038/nri2199
This article is cited by
-
Efficacy and Safety of Dupilumab in Older Patients (Aged 80 Years and Above) with Atopic Dermatitis: A Prospective Study
Drugs & Aging (2023)
-
Factors associated with the use of deworming drugs during pregnancy in Tanzania; an analysis from the 2015–16 Tanzanian HIV and malaria indicators survey
BMC Pregnancy and Childbirth (2022)
-
Tissue-based IL-10 signalling in helminth infection limits IFNγ expression and promotes the intestinal Th2 response
Mucosal Immunology (2022)
-
Investigating the development of diarrhoea through gene expression analysis in sheep genetically resistant to gastrointestinal helminth infection
Scientific Reports (2022)
-
Lessons from helminths: what worms have taught us about mucosal immunology
Mucosal Immunology (2022)