Key Points
-
Reorganization of the T-cell cytoskeleton is a rapid and dynamic process that is required to establish T-cell polarity and regulate cell–cell adhesion and T-cell activation.
-
Actin polymerization leading to lamellipodium and distal-pole formation facilitates T-cell–antigen-presenting-cell (APC) recognition.
-
Actin cytoskeletal reorganization in T cells is dependent on the dynamic interplay between actin regulatory, nucleating, capping and severing proteins.
-
Although Wiskott–Aldrich syndrome protein (WASP) and WASP-family verprolin-homologous protein-2 (WAVE2) regulate actin-related protein 2/3 (ARP2/3)-dependent actin polymerization in T cells, they clearly regulate distinct aspects of T-cell activation and filamentous (F)-actin-dependent events.
-
Microtubule-organizing centre (MTOC) polarization occurs in response to T-cell receptor (TCR) engagement and regulates directed release of cytolytic granules. This polarization requires both TCR-stimulated signalling pathways that affect actin reorganization and movement of microtubules by microtubule-associated proteins.
-
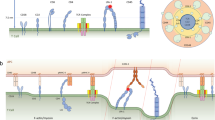
Signals arising from TCR microclusters not only induce F-actin polymerization but are themselves required for the stable formation and movement of TCR microclusters, and, ultimately, organization of the mature immunological synapse.
-
TCR-mediated integrin activation requires T-cell proximal signalling pathways that lead to cytoskeletal reorganization and the activation of RAP1. RAP1 can then act on downstream effectors including PKD, RAP1 ligand (RAPL), and RAP1-interacting adaptor protein (RIAM), leading to cytoskeletal reorganization and integrin clustering.
-
Signalling complexes that are formed at activated integrins in T cells contain proteins that are found in other cell types at focal adhesions.
-
The actin regulatory protein WAVE2 is required for integrin activation, and integrin scaffolding proteins including talin might act as linkers between newly generated F-actin and cell-surface integrins.
Abstract
To become activated, T cells must efficiently recognize antigen-presenting cells or target cells through several complex cytoskeleton-dependent processes, including integrin-mediated adhesion, immunological-synapse formation, cellular polarization, receptor sequestration and signalling. The actin and microtubule systems provide the dynamic cellular framework that is required to orchestrate these processes and ultimately contol T-cell activation. Here, we discuss recent advances that have furthered our understanding of the crucial importance of the T-cell cytoskeleton in controlling these aspects of T-cell immune recognition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vicente-Manzanares, M. & Sanchez-Madrid, F. Role of the cytoskeleton during leukocyte responses. Nature Rev. Immunol. 4, 110–122 (2004).
Sancho, D. et al. Regulation of microtubule-organizing center orientation and actomyosin cytoskeleton rearrangement during immune interactions. Immunol. Rev. 189, 84–97 (2002).
Nieminen, M. et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nature Cell Biol. 8, 156–162 (2006).
Brown, M. J., Hallam, J. A., Colucci-Guyon, E. & Shaw, S. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J. Immunol. 166, 6640–6646 (2001).
Brown, M. J., Hallam, J. A., Liu, Y., Yamada, K. M. & Shaw, S. Cutting edge: integration of human T lymphocyte cytoskeleton by the cytolinker plectin. J. Immunol. 167, 641–645 (2001). References 3, 4 and 5 describe a role for intermediate filaments in leukocyte function, and indicate that vimentin is an important structural component of the cytoskeleton during adhesion and migration.
Bunnell, S. C., Kapoor, V., Trible, R. P., Zhang, W. & Samelson, L. E. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity 14, 315–329 (2001).
Tskvitaria-Fuller, I., Rozelle, A. L., Yin, H. L. & Wulfing, C. Regulation of sustained actin dynamics by the TCR and costimulation as a mechanism of receptor localization. J. Immunol. 171, 2287–2295 (2003).
Valitutti, S., Dessing, M., Aktories, K., Gallati, H. & Lanzavecchia, A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J. Exp. Med. 181, 577–584 (1995).
Kupfer, A. & Dennert, G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J. Immunol. 133, 2762–6 (1984).
Kupfer, A., Swain, S. L. & Singer, S. J. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J. Exp. Med. 165, 1565–1580 (1987).
Cullinan, P., Sperling, A. I. & Burkhardt, J. K. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol. Rev. 189, 111–122 (2002).
Krummel, M. F., Sjaastad, M. D., Wulfing, C. & Davis, M. M. Differential clustering of CD4 and CD3ζ during T cell recognition. Science 289, 1349–1352 (2000).
Cemerski, S. & Shaw, A. Immune synapses in T-cell activation. Curr. Opin. Immunol. 18, 298–304 (2006).
Saito, T. & Yokosuka, T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr. Opin. Immunol. 18, 305–313 (2006).
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Irvine, D. J., Purbhoo, M. A., Krogsgaard, M. & Davis, M. M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Manes, S. & Viola, A. Lipid rafts in lymphocyte activation and migration. Mol. Membr. Biol. 23, 59–69 (2006).
Shaw, A. S. Lipid rafts: now you see them, now you don't. Nature Immunol. 7, 1139–1142 (2006).
Dustin, M. L. A dynamic view of the immunological synapse. Semin. Immunol. 17, 400–410 (2005).
Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Stinchcombe, J. C., Majorovits, E., Bossi, G., Fuller, S. & Griffiths, G. M. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 443, 462–465 (2006).
Varma, R., Campi, G., Yokosuka, T., Saito, T. & Dustin, M. L. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity 25, 117–127 (2006).
Wulfing, C., Sjaastad, M. D. & Davis, M. M. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc. Natl Acad. Sci. USA 95, 6302–6307 (1998).
Bunnell, S. C. et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158, 1263–1275 (2002).
Moss, W. C., Irvine, D. J., Davis, M. M. & Krummel, M. F. Quantifying signaling-induced reorientation of T cell receptors during immunological synapse formation. Proc. Natl Acad. Sci. USA 99, 15024–15029 (2002).
Caplan, S. & Baniyash, M. Searching for significance in TCR-cytoskeleton interactions. Immunol. Today 21, 223–228 (2000).
Rozdzial, M. M., Malissen, B. & Finkel, T. H. Tyrosine-phosphorylated T cell receptor ζ-chain associates with the actin cytoskeleton upon activation of mature T lymphocytes. Immunity 3, 623–633 (1995).
Badour, K., Zhang, J. & Siminovitch, K. A. The Wiskott–Aldrich syndrome protein: forging the link between actin and cell activation. Immunol. Rev. 192, 98–112 (2003).
Campi, G., Varma, R. & Dustin, M. L. Actin and agonist MHC–peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202, 1031–1036 (2005). This paper, along with references 22 and 80, describes the formation of dynamic and highly motile TCR microclusters at the periphery of the cSMAC, and indicate that they serve as platforms for active signalling during TCR–peptide–MHC interaction. Surprisingly, TCR microclusters move towards the cSMAC from the periphery, and TCR microclusters within the cSMAC were shown not to be associated with signalling molecules, which supports the possibility that the cSMAC is involved in receptor internalization and signal attenuation.
Turner, M. & Billadeau, D. D. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nature Rev. Immunol. 2, 476–486 (2002).
Wulfing, C., Bauch, A., Crabtree, G. R. & Davis, M. M. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte–antigen-presenting cell interface. Proc. Natl Acad. Sci. USA 97, 10150–10155 (2000).
Kinashi, T. Intracellular signalling controlling integrin activation in lymphocytes. Nature Rev. Immunol. 5, 546–559 (2005). Provides an in-depth analysis of many of the current paradigms involved with inside-out signalling leading to integrin activation in lymphocytes.
Morgan, M. M. et al. Superantigen-induced T cell:B cell conjugation is mediated by LFA-1 and requires signaling through Lck, but not ZAP-70. J. Immunol. 167, 5708–5718 (2001).
Dombroski, D. et al. Kinase-independent functions for ITK in TCR-induced regulation of VAV and the actin cytoskeleton. J. Immunol. 174, 1385–1392 (2005).
Labno, C. M. et al. ITK functions to control actin polymerization at the immune synapse through localized activation of CDC42 and WASP. Curr. Biol. 13, 1619–1624 (2003).
Bubeck Wardenburg, J. et al. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity 9, 607–616 (1998).
Zeng, R. et al. SLP-76 coordinates NCK-dependent Wiskott–Aldrich syndrome protein recruitment with VAV-1/CDC42-dependent Wiskott–Aldrich syndrome protein activation at the T cell–APC contact site. J. Immunol. 171, 1360–1368 (2003).
Anton, I. M. et al. WIP deficiency reveals a differential role for WIP and the actin cytoskeleton in T and B cell activation. Immunity 16, 193–204 (2002).
Badour, K. et al. The Wiskott–Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity 18, 141–154 (2003).
Tybulewicz, V. L. VAV-family proteins in T-cell signalling. Curr. Opin. Immunol. 17, 267–274 (2005).
Gomez, T. S. et al. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity 24, 741–752 (2006).
Billadeau, D. D. & Burkhardt, J. K. Regulation of cytoskeletal dynamics at the immune synapse: new stars join the actin troupe. Traffic 7, 1451–1460 (2006).
Faix, J. & Grosse, R. Staying in shape with formins. Dev. Cell 10, 693–706 (2006).
Ardouin, L. et al. VAV1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur. J. Immunol. 33, 790–797 (2003).
Villalba, M. et al. Translocation of PKCθ in T cells is mediated by a nonconventional, PI3-K- and VAV-dependent pathway, but does not absolutely require phospholipase C. J. Cell Biol. 157, 253–263 (2002).
Villalba, M. et al. VAV1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J. Cell Biol. 155, 331–338 (2001).
Faure, S. et al. ERM proteins regulate cytoskeleton relaxation promoting T cell–APC conjugation. Nature Immunol. 5, 272–279 (2004).
Charrin, S. & Alcover, A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front. Biosci. 11, 1987–1997 (2006).
Roumier, A. et al. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity 15, 715–728 (2001).
Tskvitaria-Fuller, I. et al. Specific patterns of CDC42 activity are related to distinct elements of T cell polarization. J. Immunol. 177, 1708–1720 (2006).
Cannon, J. L. & Burkhardt, J. K. Differential roles for Wiskott–Aldrich syndrome protein in immune synapse formation and IL-2 production. J. Immunol. 173, 1658–1662 (2004).
Krawczyk, C. et al. VAV1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity 16, 331–343 (2002).
Nolz, J. C. et al. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr. Biol. 16, 24–34 (2006). Using RNAi and Abi1/Abi2 -knockout mice, this article and reference 55 were the first to characterize the function of the WAVE2 complex in T cells.
Zhang, J. et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott–Aldrich syndrome protein-deficient lymphocytes. J. Exp. Med. 190, 1329–1342 (1999).
Zipfel, P. A. et al. Role for the ABI/WAVE protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr. Biol. 16, 35–46 (2006).
Gomez, T. S. et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nature Immunol. 6, 261–270 (2005).
Su, I. H. et al. Polycomb group protein EZH2 controls actin polymerization and cell signaling. Cell 121, 425–436 (2005). This study indicates that cytosolic EZH2 methyltransferase complexes associate with VAV1 and control receptor-induced actin polymerization in a methylation-dependent manner in T cells, indicating that lysine methylation of some actin regulators might be crucial for actin remodelling.
Han, J. et al. HIP-55 is important for T-cell proliferation, cytokine production, and immune responses. Mol. Cell. Biol. 25, 6869–6878 (2005).
Le Bras, S. et al. Recruitment of the actin-binding protein HIP-55 to the immunological synapse regulates T cell receptor signaling and endocytosis. J. Biol. Chem. 279, 15550–15560 (2004).
Round, J. L. et al. DLGH1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J. Exp. Med. 201, 419–430 (2005).
Ludford-Menting, M. J. et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity 22, 737–748 (2005). This article provides evidence that protein complexes previously shown to regulate polarity in epithelial cells are also present in T cells, and have a fundamental role in T-cell polarization.
Barda-Saad, M. et al. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nature Immunol. 6, 80–89 (2005).
Foger, N., Rangell, L., Danilenko, D. M. & Chan, A. C. Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis. Science 313, 839–142 (2006). This is the first study characterizing T cells from coronin-1-deficient mice, indicating that coronin-1 inhibits ARP2/3-driven actin polymerization leading to defects in migration and cell survival.
Eibert, S. M. et al. Cofilin peptide homologs interfere with immunological synapse formation and T cell activation. Proc. Natl Acad. Sci. USA 101, 1957–1962 (2004).
Nishita, M. et al. Spatial and temporal regulation of cofilin activity by LIM kinase and Slingshot is critical for directional cell migration. J. Cell Biol. 171, 349–359 (2005).
Silacci, P. et al. Gelsolin superfamily proteins: key regulators of cellular functions. Cell. Mol. Life Sci. 61, 2614–2623 (2004).
Geiger, B., Rosen, D. & Berke, G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J. Cell Biol. 95, 137–143 (1982).
Kuhn, J. R. & Poenie, M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity 16, 111–121 (2002).
Kuhne, M. R. et al. Linker for activation of T cells, ζ-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J. Immunol. 171, 860–866 (2003).
Lowin-Kropf, B., Shapiro, V. S. & Weiss, A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J. Cell Biol. 140, 861–871 (1998).
Martin-Cofreces, N. B. et al. Role of FYN in the rearrangement of tubulin cytoskeleton induced through TCR. J. Immunol. 176, 4201–4207 (2006).
Stowers, L., Yelon, D., Berg, L. J. & Chant, J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc. Natl Acad. Sci. USA 92, 5027–5031 (1995).
Kupfer, A., Mosmann, T. R. & Kupfer, H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc. Natl Acad. Sci. USA 88, 775–779 (1991).
Reichert, P., Reinhardt, R. L., Ingulli, E. & Jenkins, M. K. Cutting edge: in vivo identification of TCR redistribution and polarized IL-2 production by naive CD4 T cells. J. Immunol. 166, 4278–4281 (2001).
Faroudi, M. et al. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. Proc. Natl Acad. Sci. USA 100, 14145–14150 (2003).
Combs, J. et al. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl Acad. Sci. USA 103, 14883–14888 (2006).
Serrador, J. M. et al. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity 20, 417–428 (2004). This paper shows that HDAC6 is activated downstream of the TCR and is essential for MTOC polarity and immunological-synapse formation through its effects on microtubule stability.
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Shen, A., Puente, L. G. & Ostergaard, H. L. Tyrosine kinase activity and remodelling of the actin cytoskeleton are co-temporally required for degranulation by cytotoxic T lymphocytes. Immunology 116, 276–286 (2005). This study shows that continuous actin polymerization and depolymerization is required for ongoing tyrosine kinase activity, which leads to degranulation by CTLs.
Yokosuka, T. et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of ZAP70 and SLP-76. Nature Immunol. 6, 1253–1262 (2005).
Lee, K. H. et al. The immunological synapse balances T cell receptor signaling and degradation. Science 302, 1218–1222 (2003).
Paccani, S. R. et al. Defective VAV expression and impaired F-actin reorganization in a subset of patients with common variable immunodeficiency characterized by T-cell defects. Blood 106, 626–634 (2005). Describes a subset of T-cell defective patients with CVID whose syndrome is caused by the loss of expression of the RHO-family GEF and adaptor molecule VAV1. Patients showed loss of TCR-induced cell signalling, F-actin reorganization and upregulation of the lipid-raft constituent GM1 following TCR–CD28 engagement.
Costello, P. S. et al. The Rho-family GTP exchange factor VAV is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κB pathways. Proc. Natl Acad. Sci. USA 96, 3035–3040 (1999).
Miletic, A. V. et al. VAV1 acidic region tyrosine 174 is required for the formation of T cell receptor-induced microclusters and is essential in T cell development and activation. J. Biol. Chem. 281, 38257–38265 (2006).
Reynolds, L. F. et al. VAV1 transduces T cell receptor signals to the activation of phospholipase C-γ via phosphoinositide 3-kinase-dependent and-independent pathways. J. Exp. Med. 195, 1103–1114 (2002).
Dustin, M. L. & Springer, T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 341, 619–624 (1989).
Mittelbrunn, M. et al. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc. Natl Acad. Sci. USA 101, 11058–11063 (2004).
Goda, S., Quale, A. C., Woods, M. L., Felthauser, A. & Shimizu, Y. Control of TCR-mediated activation of β1 integrins by the ZAP-70 tyrosine kinase interdomain B region and the linker for activation of T cells adapter protein. J. Immunol. 172, 5379–5387 (2004).
Katagiri, K., Shimonaka, M. & Kinashi, T. RAP1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-γ1. J. Biol. Chem. 279, 11875–11881 (2004).
Woods, M. L. et al. A novel function for the TEC family tyrosine kinase ITK in activation of β1 integrins by the T-cell receptor. EMBO J. 20, 1232–1244 (2001).
Finkelstein, L. D., Shimizu, Y. & Schwartzberg, P. L. TEC kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J. Immunol. 175, 5923–5930 (2005).
Fischer, K. D. et al. VAV is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8, 554–562 (1998).
Schwartzberg, P. L., Finkelstein, L. D. & Readinger, J. A. TEC-family kinases: regulators of T-helper-cell differentiation. Nature Rev. Immunol. 5, 284–295 (2005).
Boussiotis, V. A., Freeman, G. J., Berezovskaya, A., Barber, D. L. & Nadler, L. M. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated RAP1. Science 278, 124–128 (1997).
Sebzda, E., Bracke, M., Tugal, T., Hogg, N. & Cantrell, D. A. RAP1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nature Immunol. 3, 251–258 (2002).
Duchniewicz, M. et al. RAP1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol. Cell. Biol. 26, 643–653 (2006).
Zhang, W. et al. Negative regulation of T cell antigen receptor-mediated CRK-L-C3G signaling and cell adhesion by CBL-B. J. Biol. Chem. 278, 23978–23983 (2003).
Peterson, A. C., Marks, R. E., Fields, P. E., Imamoto, A. & Gajewski, T. F. T cell development and function in CRKL-deficient mice. Eur. J. Immunol. 33, 2687–2695 (2003).
Katagiri, K., Maeda, A., Shimonaka, M. & Kinashi, T. RAPL, a RAP1-binding molecule that mediates RAP1-induced adhesion through spatial regulation of LFA-1. Nature Immunol. 4, 741–748 (2003).
Katagiri, K., Imamura, M. & Kinashi, T. Spatiotemporal regulation of the kinase MST1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nature Immunol. 7, 919–928 (2006). This report identifies the serine/threonine kinase MST1 as a RAP1/RAPL effector and shows the importance of this protein in regulating TCR-mediated integrin activation.
Medeiros, R. B. et al. Protein kinase D1 and the β1 integrin cytoplasmic domain control β1 integrin function via regulation of RAP1 activation. Immunity 23, 213–226 (2005).
Lafuente, E. M. et al. RIAM, an Ena/VASP and Profilin ligand, interacts with RAP1–GTP and mediates RAP1-induced adhesion. Dev. Cell 7, 585–595 (2004).
Han, J. et al. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr. Biol. 16, 1796–1806 (2006).
Wang, H. et al. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nature Immunol. 4, 366–374 (2003).
Griffiths, E. K. et al. Positive regulation of T cell activation and integrin adhesion by the adapter FYB/SLAP. Science 293, 2260–2263 (2001).
Peterson, E. J. et al. Coupling of the TCR to integrin activation by SLAP-130/FYB. Science 293, 2263–2265 (2001).
Kliche, S. et al. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of RAP1. Mol. Cell. Biol. 26, 7130–7144 (2006).
Marie-Cardine, A. et al. Molecular interaction between the FYN-associated protein SKAP55 and the SLP-76-associated phosphoprotein SLAP-130. J. Biol. Chem. 273, 25789–25795 (1998).
Liu, J. et al. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc. Natl Acad. Sci. USA 95, 8779–8784 (1998).
Huang, Y. et al. Deficiency of ADAP/FYB/SLAP-130 destabilizes SKAP55 in Jurkat T cells. J. Biol. Chem. 280, 23576–23583 (2005).
Krause, M. et al. FYN-binding protein (FYB)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 149, 181–194 (2000).
Simonson, W. T., Franco, S. J. & Huttenlocher, A. Talin1 regulates TCR-mediated LFA-1 function. J. Immunol. 177, 7707–7714 (2006).
Suzuki, J. I., Yamasaki, S., Wu, J., Koretzky, G. A. & Saito, T. Actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T cell activation. Blood 109, 168–175 (2006).
Zamir, E. & Geiger, B. Molecular complexity and dynamics of cell–matrix adhesions. J. Cell Sci. 114, 3583–3590 (2001).
Critchley, D. R. Genetic, biochemical and structural approaches to talin function. Biochem. Soc. Trans. 33, 1308–1312 (2005).
Critchley, D. R. Focal adhesions — the cytoskeletal connection. Curr. Opin. Cell Biol. 12, 133–139 (2000).
DeMali, K. A., Barlow, C. A. & Burridge, K. Recruitment of the ARP2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 159, 881–891 (2002).
Tachibana, K. et al. Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase. A putative mechanism for the integrin-mediated tyrosine phosphorylation of CRK-associated substrates. J. Biol. Chem. 272, 29083–29090 (1997).
Schlaepfer, D. D. & Mitra, S. K. Multiple connections link FAK to cell motility and invasion. Curr. Opin. Genet. Dev. 14, 92–101 (2004).
Parsons, J. T. Focal adhesion kinase: the first ten years. J. Cell Sci. 116, 1409–1416 (2003).
Acknowledgements
The authors apologize to many colleagues whose exciting and important work was not quoted here owing to space constraints. We would like to thank J. Burkhardt, Y. Shimizu and P. Leibson for helpful discussions and critical review of the manuscript. This work was supported by the Mayo Foundation and grants from the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Glossary
- Immunological synapse
-
A large junctional structure that is formed at the cell surface between a T cell that is interacting with an APC; it consists of molecules required for adhesion and signalling. This structure is important in establishing T-cell adhesion and polarity, is influenced by the cytoskeleton, and transduces highly controlled secretory signals, thereby allowing the directed release of cytokines or lytic granules towards the APC or target cell.
- Actin-severing proteins
-
These proteins are activated downstream of cell-surface receptors and cleave filamentous actin, thereby promoting new actin-filament growth.
- Actin-capping proteins
-
These proteins bind to the barbed end of filamentous actin and prevent further actin polymerization.
- Actin-regulatory proteins
-
These proteins regulate actin dynamics through their associations with filamentous actin, either directly or by promoting actin nucleation through regulation of actin nucleators.
- Actin-nucleating proteins
-
These proteins can facilitate actin nucleation through binding either the plus (barbed) or minus end of F-actin in conjunction with their binding to monomeric (G)-actin or G-actin–profilin complexes.
- Microtubule-organizing centre
-
(MTOC). A structure that is found in all plant and animal cells, from which microtubules radiate. The two most important types of MTOCs are the basal bodies that are associated with cilia and the centrosome, which is composed of γ-tubulin-ring complexes for microtubule nucleation.
- Distal-pole complex
-
A rigid membrane projection with cytoskeletal components that is formed during the process of T-cell–APC recognition. It is located at the back of the cell, opposing the lamellipodium and immunological synapse and is proposed to function in polarity and as a sink for negative regulators of T-cell activation.
- Central and peripheral supramolecular activation clusters
-
(cSMAC and pSMAC). During T-cell activation, TCRs accumulate into a central cluster (known as the cSMAC) at the interface between the T cell and the APC. The cSMAC is surrounded by a leukocyte-function-associated antigen-1 (LFA1)-integrin ring (known as the pSMAC) in a bulls-eye fashion, and this characteristic receptor organization (cSMAC surrounded by the pSMAC) constitutes the mature immunological synapse.
- Lipid raft
-
Structures that are proposed to arise from phase separation of different plasma membrane lipids as a result of the selective coalescence of certain lipids on the basis of their physical properties. This results in the formation of distinct and stable lipid domains in membranes that might provide a platform for membrane-associated protein organization.
- T-cell receptor (TCR) microclusters
-
Accumulated TCR–peptide–MHC complexes that continuously form during T-cell activation and are thought to be localized sites of antigen recognition and T-cell activation.
- Lamellipodium
-
A flattened, sheet-like protrusion that is supported by a meshwork of F-actin and extends at the leading edge of crawling cells.
- ARP2/3 complex
-
The actin-related protein-2/3 (ARP2/3) complex is composed of seven proteins, including ARP2, ARP3, and the ARP complex protein-1 (ARPC1)–ARPC5. On its own, the complex has little activity but when bound to an ARP2/3-nucleation-promoting factor, it is activated to generate new actin filaments off pre-existing filaments.
- ERM family proteins
-
Ezrin, radixin and moesin (ERM) proteins act as general cytolinkers between the cortical actin-filament network and the plasma membrane, and are thought to function mainly through their ability to bind both F-actin and the cytoplasmic regions of integral membrane proteins.
- Wiskott–Aldrich syndrome
-
(WAS). A rare X-linked recessive disease that is caused by mutations in the WAS gene, and is characterized by immune dysregulation (immunodeficiency, eczema and autoimmunity) and thrombocytopaenia (decreased platelet number).
- RNA interference
-
(RNAi). RNAi is a method of post-transcriptional control of gene expression, in which the introduction of small, sequence-specific, double-stranded RNAs into cells leads to the degradation of mRNAs that have a complementary sequence.
- Minus end
-
The slow-growing end of a microtubule, which is anchored to the centrosome.
- Total internal reflection fluorescence microscopy
-
(TIRFM). This microscopy method allows the identification of fluorescence within 100–200 nm of the interface between cells and their substrate (for example, lipid bilayers or glass coverslips), thereby providing a high lateral and axial resolution at the cell–substrate interface and the ability to observe nanoscale movement of signalling molecules during cellular activation.
- Common variable immunodeficiency syndrome
-
(CVID). The most common symptomatic primary antibody deficiency, which is characterized by decreased levels of serum immunoglobulin and a low or normal number of B cells.
- Calcium-regulated activated calcium (CRAC) channels
-
In several cell types, receptor-mediated signalling that leads to the depletion of endoplasmic-reticulum intracellular stores of calcium by PLCγ1-generated IP3 results in capacitive calcium entry from the outside of the cell through CRAC channels.
- Inside-out signalling
-
The process by which intracellular signalling mechanisms result in the activation of a cell-surface receptor. By contrast, outside-in signalling is the process by which ligation of a cell-surface receptor activates signalling pathways inside the cell.
- Extravasation
-
The cellular process in which circulating leukocytes bind to and migrate through the endothelium into the underlying tissue.
- Phorbol 12-myristate 13-acetate
-
(PMA). PMA is a synthetic diacylglycerol analogue and potent pharmacological agent that is often used to stimulate activation of PKC-family members independently of receptor stimulation.
Rights and permissions
About this article
Cite this article
Billadeau, D., Nolz, J. & Gomez, T. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol 7, 131–143 (2007). https://doi.org/10.1038/nri2021
Issue Date:
DOI: https://doi.org/10.1038/nri2021
This article is cited by
-
Motility and tumor infiltration are key aspects of invariant natural killer T cell anti-tumor function
Nature Communications (2024)
-
LFA-1 knockout inhibited the tumor growth and is correlated with treg cells
Cell Communication and Signaling (2023)
-
Lack of Nck1 protein and Nck-CD3 interaction caused the increment of lipid content in Jurkat T cells
BMC Molecular and Cell Biology (2022)
-
Transient 40 °C-shock potentiates cytotoxic responses of Vδ2+ γδ T cell via HSP70 upregulation
Cancer Immunology, Immunotherapy (2022)
-
The DNA-helicase HELLS drives ALK− ALCL proliferation by the transcriptional control of a cytokinesis-related program
Cell Death & Disease (2021)