Key Points
-
Needles and syringes are the most commonly used method for administering vaccines. They are responsible for numerous cases of transmission of HIV, hepatitis B virus and hepatitis C virus, which have arisen from the reuse of needles and syringes. Development of needle-free methods of immunization is a high priority.
-
Current options for needle-free immunization can be classified into four categories: liquid-jet injection, epidermal powder immunization, and topical application (which are all types of cutaneous immunization), and mucosal immunization.
-
Liquid-jet injection has been used for immunization for more than 50 years. Multi-use-nozzle jet injectors have been used for mass immunization, but this practice was discontinued because of concerns about cross-contamination. At present, disposable-cartridge jet injectors are used for immunization in clinics.
-
Epidermal powder immunization delivers dry particulate vaccine to the epidermis. It effectively targets Langerhans cells.
-
Topical vaccine application is being developed for immunization. Various methods — including topical adjuvant patches, colloidal carriers, and physical methods, such as ultrasound, microneedles, tape stripping and thermal microporation — are being used to improve vaccine penetration of the skin and to provide adjuvant activity.
-
During mucosal immunization, vaccines are delivered through the ocular, oral, nasal, pulmonary, vaginal or rectal route. Various methods — including the use of adjuvants (such as the cholera toxin B subunit and bacterial ghosts) and the encapsulation of vaccines in carriers (such as liposomes and microspheres) — are being developed for mucosal immunization.
-
In the past decade, considerable progress has been made towards needle-free immunization. At present, several companies are developing needle-free methods of immunization.
Abstract
Most current immunization procedures make use of needles and syringes for vaccine administration. With the increase in the number of immunizations that children around the world routinely receive, health organizations are beginning to look for safer alternatives that reduce the risk of cross-contamination that arises from needle reuse. This article focuses on contemporary developments in needle-free methods of immunization, such as liquid-jet injectors, topical application to the skin, oral pills and nasal sprays.
Similar content being viewed by others
Main
Needles and syringes are the most commonly used method for administering vaccines and protein therapeutics, such as insulin, to humans. The World Health Organization (WHO; see the Online links box) estimates that 12 billion injections are given annually, 5% of which are used for immunizations1. Despite their common use, needle-based immunizations have several limitations. Needle phobia is an important issue for both adults and children2 and makes immunizations stressful3. In addition, accidental needle sticks are a serious problem in both developed and developing countries. The Centers for Disease Control and Prevention (CDC; see National Immunization Program in the Online links box), in the United States, estimates that more than 300,000 needle-stick injuries occur annually in US hospitals4. An estimated 5 accidental needle-stick injuries occur per 100 injections worldwide, posing a considerable risk to health-care providers5.
An even greater shortcoming of injections is their improper and unsafe use. This mainly involves the reuse of needles and syringes, which is common in developing countries for reasons of cost1. (A detailed list of unsafe injection practices is given in Ref. 1.) The WHO has estimated that as many as one-third of immunization injections are unsafe in four of its six geographical regions5,6. Each year, an overwhelming number of infections with HIV (80,000–160,000), hepatitis C virus (HCV; 2.3–4.7 million) and hepatitis B virus (HBV; 8–16 million) are thought to originate from the reuse of needles and syringes by health-care providers7. The WHO estimates that 32% of HBV infections, 40% of HCV infections and 5% of HIV infections in developing countries are attributable to unsafe injection practices8. Not surprisingly, the development of needle-free immunization methods has now been identified as an important goal in global health care9.
Needle-free immunizations made their first notable appearance almost 50 years ago with the oral polio vaccine, which is still used in developing countries but has been discontinued in the United States since 2000 (Box 1). This vaccine, which contains a LIVE ATTENUATED poliovirus, can infect the gastrointestinal tract and, subsequently, generate adequate immune protection in the host. Several other needle-free vaccines (oral typhoid fever, oral cholera, oral rotavirus and nasal influenza), which also contain live attenuated pathogens, are now available (Timeline). However, the administration of most vaccines without the use of needles has proved to be challenging, especially for non-living vaccines (that is, killed pathogens, and subunit, toxoid, peptide and DNA vaccines), which offer several advantages (Box 1). Consequently, in developed countries, as well as developing countries, most childhood vaccines — including those against hepatitis B (a subunit vaccine), diphtheria–tetanus–pertussis (toxoid and inactivated bacteria), polio (killed virus), varicella (live attenuated virus), measles–mumps–rubella (live attenuated virus), tuberculosis (live attenuated bacteria) and yellow fever (live attenuated virus) — are administered using needles and syringes. In the past decade, however, there has been a strong step forward in addressing the technological challenges that are associated with immunization without needles10,11.
Current methods of needle-free immunization, either commercially available or under development, can be classified into two broad classes — cutaneous immunization and mucosal immunization — depending on the site of vaccine administration (Fig. 1). Cutaneous methods of immunization include the following: liquid-jet injection, which delivers a high-speed vaccine stream into intradermal, subcutaneous or intramuscular regions; ballistic methods (also known as epidermal powder immunization), which accelerate particulate vaccine material and deposit it in the skin; and topical application methods, which deliver the vaccine into or across the skin through passive diffusion or facilitated transdermal transport (Fig. 2). Mucosal immunization methods involve delivery of vaccines to a mucosal membrane, such as the ocular, oral, nasal, pulmonary, vaginal or rectal membrane (Table 1). This article provides an overview of the development of these methods, with an emphasis on the challenges that are associated with the delivery of non-living vaccines. Particular attention is paid to needle-free cutaneous immunization. Detailed discussion of mucosal immunization can be found elsewhere11,12.
Four distinct modes of immunization are discussed in this article: liquid-jet injection, epidermal powder immunization and topical application (which are all forms of cutaneous immunization), and mucosal immunization, which is further classified into ocular, nasal, oral, pulmonary, and vaginal or rectal routes. Ocular immunization can be carried out using eye drops. Nasal immunization is carried out using sprays that comprise liquid formulations, liposomes or microspheres. Vaccines can be delivered orally in the form of liquid doses or pills, both of which can consist of various formulations: for example, microspheres. Vaccines can also be delivered to the vaginal or rectal mucosal membrane, using topical creams, or to the lungs, using aerosols or powders. Liquid-jet injection delivers vaccines to the skin, subcutaneous fat or muscles, depending on the parameters of the injection. Epidermal powder immunization delivers vaccine powders to the superficial layers of the skin. Transdermal patches also deliver vaccines to the superficial layers of the skin. Topical delivery of vaccines is facilitated by adjuvant patches, colloidal carriers, or physical methods, such as microneedles and ultrasound. For a general discussion of the issues that are associated with delivery of molecules (not just vaccines) through these routes, see the following references: ocular drug delivery148,149, epidermal powder delivery150, liquid-jet injection23, transdermal drug delivery51, nasal drug delivery151,152, pulmonary drug delivery153, oral drug delivery86,154 and vaginal drug delivery155.
A | Liquid-jet injection delivers vaccine to muscular, subcutaneous or dermal regions, depending on the parameters of the injection. B | Epidermal powder immunization delivers vaccine powders to the superficial layers of the skin (that is, the epidermis and the superficial layers of the dermis), where they are recognized by Langerhans cells. C | Topical application of vaccines delivers vaccines to the epidermis, where they are recognized and processed by Langerhans cells. Immunization by topical vaccine application is facilitated by several methods. Ca | DNA immunization can be carried out through hair follicles. Cb | Tape stripping removes the stratum corneum and facilitates vaccine absorption. Cc | Thermal or radio-wave-mediated ablation of the stratum corneum creates micropores that increase vaccine delivery. Cd | Colloidal carriers such as microemulsions and transfersomes increase dermal absorption of topically applied vaccines. Ce | Low-frequency ultrasound is an adjuvant for topically applied vaccines, and it also increases vaccine delivery to the skin. Cf | Topically applied adjuvants, such as cholera toxin, can induce potent immune responses. Cg | Electroporation of the stratum corneum increases the delivery of DNA vaccines to the epidermis. Ch | Shallow microneedles that penetrate into the epidermis deliver vaccines effectively. For an overview of the issues that are associated with transdermal delivery of molecules (not just vaccines) by some of these methods, see Refs 56, 72, 156–158.
Liquid-jet injections
Jet injection is the oldest method of needle-free immunization. The origin of jet injections can be traced to the late 1800s, when a technique known as aquapuncture was reported in the medical literature13. This device was used to deliver jets of water and other liquids for applications other than immunization: for example, for the treatment of uncontrolled neuralgia. However, it was in the early 1950s when jet injections took their place as a needle-free method of delivering medications and vaccines14.
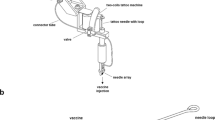
A liquid-jet injector uses the kinetic energy of a high-velocity vaccine jet (typically more than 100 m per second) with a diameter that ranges from 76 μm to 360 μm, which is smaller than the outer diameter of a standard hypodermic needle (810 μm for a 21G needle). Liquid jets penetrate the skin and deliver the vaccine into the skin (that is, intradermally), the subcutaneous tissue or the muscle (intramuscularly) (Fig. 2A). Skin is a particularly attractive target for vaccine administration because it forms an integral part of the immune system15,16. The epidermis is enriched with LANGERHANS CELLS, which form a network that allows them to take up antigen efficiently and therefore to carry out immune surveillance17. The Langerhans-cell network is the next line of defence after the physical barrier of the skin has been breached. Langerhans cells initiate specific immune responses by processing and presenting antigen fragments to naive T cells in the lymph nodes18. This promotes the generation of both systemic (IgG and IgM) and mucosal (IgA) humoral immune responses19,20. Targeting the vaccine to the skin promotes its contact with Langerhans cells and reduces the required dose of vaccine21,22, a factor that would become crucial at a time of vaccine shortage, such as the predicted H5N1 influenza-virus pandemic. Vaccines that are delivered by liquid-jet injectors typically spread throughout a larger tissue volume after injection than do vaccines that are administered with needles23, which might allow them to establish better or faster contact with antigen-presenting cells before they are degraded.
Liquid-jet injections were first popularized by multi-use-nozzle jet injectors (MUNJIs), which allow injection of several doses using the same nozzle and vaccine reservoir at a rate of up to 1,000 immunizations per hour. MUNJIs were successfully used for immunizing humans with live vaccines against measles and smallpox, as well as non-living vaccines against cholera, hepatitis B, influenza and polio24. Liquid-jet injectors offer several advantages in addition to avoiding the use of sharps. They have a long history of use, and they work with existing vaccine formulations that have been developed for needle-based administration. In one example, they resulted in higher SEROCONVERSION rates, but the reasons for this are not clear25. At the same time, liquid-jet injectors have several limitations. In some studies, they were associated with higher levels of pain than were needle-based injections26, especially when using older MUNJI devices. Liquid-jet injectors have also been associated with more-frequent site reactions than have needles, such as soreness, redness and swelling of the injection site25,27.
Perhaps the main safety issue that is associated with MUNJIs is the increased risk of subject-to-subject contamination. In the 1980s, the spread of HBV between subjects was linked to liquid-jet injections28. Splashes of small amounts of blood or interstitial fluid on the nozzle of the MUNJI were blamed for this spread of HBV. Systematic studies have shown that MUNJIs can transmit considerable volumes of blood (more than 10 pl) from one subject to another when the MUNJIs are used on multiple subjects. This volume of blood is presumed to be sufficient to transmit HBV infection29. The WHO and CDC recommend that MUNJIs should be used for mass immunization only when the gains from rapid immunization outweigh the risks of blood-borne disease: for example, during influenza pandemics or bioterrorism attacks30. To minimize the risk of contamination, protective devices that are disposable have been developed to cover the surface of the injector, and studies that have been carried out with these devices showed no risk of contamination31. Disposable-cartridge jet injectors (DCJIs; which are non-disposable injectors to which disposable nozzles are attached for each use) have also been developed to alleviate concerns about contamination. Single-use, pre-filled disposable devices are also under development to alleviate these concerns about contamination, and these devices present a new direction in liquid-jet injectors32. In addition to conventional vaccines, some DCJIs have also been effectively used to carry out DNA vaccination against dengue fever and influenza in animals33,34,35.
Although MUNJIs are no longer used for routine immunizations, DCJIs are used for childhood immunizations at the physician's discretion. However, at present, the number of immunizations that is carried out with DCJIs is far less than the number that is carried out using needles, possibly because of the cost, the low level of awareness among health-care providers and patients, the potential pain, and the problems that were associated with earlier generations of liquid-jet injectors.
Numerous liquid-jet injectors are already on the market and are used for various vaccines, including those against influenza and hepatitis B. Newer, more convenient liquid-jet injectors are continually being developed, mainly by small companies. Although substantial technological advances have been made in the past decade, the fundamental science that underlies liquid-jet injections is not well understood. Studies on various basic aspects of liquid-jet injections, such as dynamics of jet fluid, mechanics of jet penetration and dynamics of jet dispersion, have only recently been reported in the literature36,37. It is hoped that this understanding, together with technological advances, will lead to better and cheaper devices.
Particle bombardment of the skin
Particle-based methods (also known as ballistic methods) accelerate powdered vaccines such that they penetrate the outer layer of the skin (that is, the stratum corneum) and are deposited in the epidermis or the superficial layers of the dermis, a method known as epidermal powder immunization (EPI)38 (Fig. 2B). The ballistic technique was first developed in 1986, for the delivery of DNA-coated metal particles of ∼1 μm in diameter into plants to genetically modify them, and it was known as the gene gun. In the early 1990s, the ballistic method was developed into devices for delivering both conventional and DNA vaccines to humans39. Unlike liquid-jet injectors, which routinely deliver the vaccine to the subcutaneous or intramuscular space, ballistic methods mainly deliver the vaccine to the superficial layers of the skin39and therefore naturally target Langerhans cells.
Several vaccines have been delivered to animals using EPI. Influenza vaccine, when administered together with ADJUVANTS such as cholera toxin (CT) by EPI, elicited augmented serum and mucosal antibody responses in mice compared with those in unimmunized animals40,41. Similar results were reported for diphtheria toxoid (DT). Co-administration of adjuvants increased IgG titres after EPI-based immunization against diphtheria42 and influenza43. EPI has also been used to deliver DNA vaccines to animals. Small (1–3 μm) DNA-coated gold or tungsten particles delivered by EPI directly penetrate epidermal keratinocytes or Langerhans cells, and they induce expression of encoded antigens44. Additional means to capitalize on the immunostimulatory properties of Langerhans cells — for example, co-administering DNA that encodes cytokines (such as interleukin-6) or inhibitors of apoptosis45 — have also been adopted. Apoptosis inhibitors increase the survival of Langerhans cells after particle-induced mechanical trauma, and the expression of cytokines facilitates the migration of Langerhans cells, which is typically promoted by pro-inflammatory mediators.
There are fewer reports of EPI of humans. In one study, EPI efficiently delivered influenza vaccine to humans46. For all influenza-virus strains, titres of IgG were equivalent in groups in which EPI was used and in needle-immunized individuals. Clinical studies of immunization with DNA using ballistic methods have also yielded encouraging results47,48,49. EPI-based DNA immunization against infection with HBV induced high titres of protective antibody, as well as cell-mediated immune responses, in humans. Despite promising results in clinical studies, however, the commercial development of EPI for conventional vaccines seems to be stagnant. Instead, current development efforts in industry are focused entirely on DNA vaccines.
EPI offers several advantages as a mode of immunization. The use of powders simplifies handling and storage compared with liquid formulations. EPI also naturally targets Langerhans cells and allows their direct transfection. Initial safety studies of EPI seem to be satisfactory, although occasional bleeding was observed in some cases46. As is the case for liquid-jet injectors, fundamental studies that focus on the mechanics of particle penetration, which will be useful for understanding the mechanisms of EPI, have only recently been initiated38,50. These studies have documented the role in EPI of the properties of the particle (such as density and size), the operating conditions (that is, temperature, humidity and velocity) and the mechanical properties of the skin. Understanding gained from such studies might assist in the design of future EPI devices.
Topical application to the skin
The skin has been used for administering medication to treat local conditions (for example, inflammation) for thousands of years. Systemic drug delivery through the skin became prominent with the introduction of transdermal scopolamine patches for treating motion sickness, in 1979 (Ref. 51). The use of the skin for the administration of vaccines has an even longer history. Immunization against smallpox was practiced in India more than 1,000 years ago by scratching dry scabs from smallpox lesions onto the skin of healthy individuals. The skin remains the site for immunization against smallpox in the modern era, using the bifurcated needle52. Although the skin has had a historical role in immunization, the use of topical vaccine application as a general mode of immunization has only recently (in the mid-1990s) received attention.
The simple topical application of a vaccine does not typically yield an adequate immune response, although rare cases can be found in the literature53. Topical delivery of vaccines into the skin is limited by the low permeability of the stratum corneum, the outer layer of skin, which is 15–20 μm in thickness and consists of cornified keratinocytes embedded in a lipid-rich matrix. The lipids of the stratum corneum are organized into an ordered bilayer structure and, consequently, form a strong barrier to molecular transport54. Increasing the permeability of the stratum corneum without irritating the underlying keratinocytes has been a considerable challenge in the field. Several innovative methodologies are being developed to facilitate antigen delivery into the skin. These include the use of topically applied adjuvants, COLLOIDAL CARRIERS to encapsulate vaccines, and physical methods to increase the permeability of the skin to vaccines (Fig. 2C).
Topical adjuvants. Topical application of adjuvants such as CT together with the vaccine on the skin generates a strong systemic and mucosal immune response55,56,57. This is the most studied of all topical-immunization methods. Topical application of CT provides the required activation signal for Langerhans cells to mature and become potent antigen-presenting cells that can prime the immune response to co-administered vaccines58. It is unclear how CT, a relatively large protein (86 kDa), diffuses across the stratum corneum, but hydration-induced permeabilization of the stratum corneum is one possibility. In recent studies, however, disruption of the stratum corneum using emery paper (which is an abrasive paper) has been used before the application of vaccine and/or adjuvant to achieve immune responses59,60. Additional strategies, which also involve disruption of the stratum corneum, are discussed later.
Several other adjuvants that have fewer toxicity issues than CT, such as the B subunit of CT (CTB), also have adjuvant-like properties after topical administration61. The effectiveness of adjuvant-mediated cutaneous immunization has been shown in animals, using vaccines that include tetanus toxoid (TT)19, DT62 or Bacillus anthracis (the causative agent of anthrax)59. Clinical studies have also confirmed the generation of a strong IgG and IgA response in volunteers after topical application of a colonization factor from enterotoxigenic Escherichia coli together with adjuvants63. Earlier clinical studies that were carried out using E. coli heat-labile enterotoxin (LT) as an adjuvant showed that mucosal antibodies were generated, and these studies confirmed the role of Langerhans cells in immunization by topical vaccine application, as shown by the strong presence of these cells in the skin 24–48 hours after immunization64.
Colloidal carriers. Encapsulation of vaccines in colloidal carriers facilitates the generation of an immune response after topical application. Few studies have reported on the use of colloidal carriers for the topical delivery of vaccines, all in animals. Topical application of TT encapsulated in lipid vesicles, after booster immunization, elicited a specific immune response (IgG) comparable to that produced by intramuscular injections of alum-adsorbed TT65. Another lipid-based system has been used to deliver DNA vaccines to animals66. Topical application of this DNA–lipid vaccine resulted in both antibody responses and cellular responses. Ethanol-in-fluorocarbon MICROEMULSION systems67,68 and cationic nanoparticles coated with DNA vaccines have also been used for topical DNA immunization of animals69. The precise mechanisms by which colloidal carriers penetrate the stratum corneum remain a topic of research. Whether the results obtained from animal studies can be translated to humans also remains to be seen.
Physical methods. Physical techniques that use microneedles, tape stripping, ultrasound, microporation or electroporation have also been used to deliver vaccines across the skin. Most of these techniques, although well studied for general drug-delivery applications, have only recently emerged as potential immunization techniques. In the microporation technique, a vaporization process (which involves focused deposition of thermal energy into the skin through an electrically heated element) is used to remove small areas of the stratum corneum, thereby exposing the immunocompetent epidermis. In one study, in hairless mice, application of an adenoviral vector to microporated skin resulted in 10–100-fold greater cellular and humoral immune responses than did application to intact skin70. Microneedles (which are solid and hollow arrays of micrometre-scale silicon projections) have also been used on several occasions to carry out topical immunization with various vaccines71,72,73. Microprojection arrays have been used to deliver naked plasmid DNA, inducing stronger and less variable immune responses (as judged by serum IgG titres) than those induced by needle-based injections. They also reduced the number of immunizations that was required for full seroconversion73. In another study, microprojection-array patches were used to deliver a model antigen, ovalbumin, to generate a strong immune response71. Ovalbumin that was administered by microprojection array generated an immune response up to 50-fold greater than that observed after the same dose administered subcutaneously or intramuscularly using a needle.
A handful of studies have reported the use of tape stripping to facilitate transdermal vaccine absorption in animals74,75. Repeated peeling using tape (for example, Scotch tape) effectively removes the stratum corneum. Application of peptides that represent tumour-derived epitopes to tape-stripped mouse skin primed tumour-specific cytotoxic T cells in the lymph nodes and the spleen, protected mice against a subsequent challenge with the corresponding tumour cells and suppressed the growth of established tumours76. Skin abrasion using a razor and a toothbrush followed by application of adenoviral vectors has yielded promising results in humans77.
Ultrasound, at low frequency (20 kHz), has also been shown to deliver a vaccine (consisting of TT) to mice in one study78. The immune response that was generated by ultrasonically delivered vaccine was about tenfold greater per unit dose of vaccine that entered the skin than occurred after subcutaneous injection. (About 1% of the topically applied dose entered the skin78.) Compared with simple topical administration, pretreatment with ultrasound was shown to increase vaccine delivery, thereby allowing enough vaccine to enter the skin to activate the immune response. Ultrasound has been shown to increase skin permeability through disruption of the stratum corneum by acoustic cavitation (which involves the formation and collapse of gaseous cavities)79. Furthermore, application of ultrasound resulted in activation of Langerhans cells, the reasons for which are not clear. In another study, electroporation (which involves the application of high-voltage, short-duration electric pulses) has been used to increase the delivery of DNA vaccines across the skin of mice80. Electroporation has also been shown to induce an effective immune response after transdermal delivery of peptide vaccines81. Additional approaches, including the use of laser-assisted permeabilization of the stratum corneum and various designs of microneedles (other than those published in peer-reviewed literature), are also being pursued by industry. Most of the physical methods for topical immunization have only been tested on animals, specifically on mice or rats. It remains to be seen how many of these methods can be applied to human skin, which differs substantially in barrier properties from rodent skin. Some of the methods that are discussed in this section are purported to have been tested on humans for immunization purposes; however, these studies have not yet appeared in peer-reviewed literature.
Topical vaccine application (including the use of topical adjuvants, colloidal carriers and physical disruption) offers several advantages. Administration to the skin is generally easy to carry out and leads to high compliance of patients82. Topical vaccine application also naturally targets Langerhans cells. However, cost issues must be investigated in depth before these techniques can be adopted for wide-scale applications in humans. Methods that are based on physical techniques such as ultrasound, electroporation and microporation use expensive devices, which might pose constraints on their adoption by developing countries. The use of electric power might limit the widespread use of some of these methods, especially for field applications. Several companies, mostly small-scale businesses, are working to address these challenges.
Mucosal administration
Mucosal routes (especially oral and nasal routes) have been used for delivering medication for millennia, pre-dating needles and syringes. Several centuries ago, nasal administration of dried scabs of smallpox lesions and oral administration of fleas from cows with cowpox were practiced in China as a means of immunization against smallpox. It was the Sabin oral polio vaccine, however, that brought mucosal immunization to prominence, in the early 1960s, and that had an important role in the programme for global eradication of polio. Since then, several mucosal vaccines have been marketed (Timeline). Because many pathogens — for example, HIV and influenza virus — enter the body through mucosal tissues, the development of vaccines that offer mucosal immunity has received considerable attention in the past 20 years12,83.
Oral route. Oral delivery of vaccines is an attractive mode of immunization because of its acceptability and its ease of administration84. Orally delivered vaccines, especially particulates, are recognized by MICROFOLD (M) CELLS (which sample antigen) in the PEYER'S PATCHES of the intestine and by dendritic cells that reside there12 (Fig. 3). At present, few vaccines (those against polio, typhoid fever and cholera) are administered orally, and most of these are based on live attenuated pathogens (Timeline). Oral delivery of non-living vaccines has proved to be extremely challenging, owing to poor stability of proteins, peptides and DNA in the acidic and enzyme-rich environment of the gastrointestinal tract85. Several strategies, including the use of biodegradable polymeric particles and LIPOSOMES, have been adopted to protect the antigens in the gastrointestinal tract86,87. In addition, strong adjuvants — for example, bacterial enterotoxins such as CT and LT — have also been successfully used for the oral immunization of animals88. However, toxicity of these enterotoxins limits their applications in humans89. To alleviate the toxicity issues, mutants and subunits of LT and CT have been used as adjuvants in many studies of oral immunization of animals90. A detailed review of mucosal adjuvants can be found in Refs 88, 89.
Vaccines that are delivered by mucosal routes (that is, ocular, oral, nasal, pulmonary, vaginal or rectal routes) are recognized by microfold (M) cells and dendritic cells in the mucosa-associated lymphoid tissue. Particulate antigens (such as microspheres, liposomes and bacterial ghosts) are recognized by receptors at the surface of M cells and are presented to lymphocytes and macrophages. Soluble antigens, as well as small pathogens, can permeate the epithelium and be recognized by dendritic cells.
Encapsulation of antigens in biodegradable polymer microspheres (especially poly(lactide-co-glycolide), PLG) has been successfully used for oral immunization of animals against HBV91, TT92 and other antigens86,93. Several other materials have also been used to encapsulate antigens, but a clear advantage of any particular material is not obvious94,95,96. In addition to protecting antigens from the hostile gastrointestinal environment, microspheres have been suggested to aid immunization through the sustained release of antigens, which might overcome the need for booster doses, which are typically required for vaccines that are administered by intramuscular injection97. Additional mechanisms, such as direct intracellular delivery of antigens through phagocytosis of particles, have also been proposed to explain the adjuvant activity of polymeric microspheres98. Oral delivery of DNA vaccines that have been encapsulated in PLG or chitosan microspheres has also received considerable attention99,100,101. Several strategies that involve the use of antibodies, IgA or lectins have also been proposed to target microspheres to M cells102.
Liposomes offer an alternative means of protecting a vaccine103,104,105. Conventional liposomes are not particularly stable in the gastric environment, so polymerized liposomes have been developed as carriers for oral vaccines106. Modification of liposome composition using lipids from archaebacteria has been attempted to facilitate vaccine uptake by antigen-presenting cells107,108. Both nanoscale lipid particles that consist of lipids, adjuvants and antigens, which are known as immunostimulating complexes (ISCOMs)107, and BACTERIAL GHOSTS109, which are bacteria that lack their cytoplasmic contents, have also been successfully used as vaccine carriers in animals. Bacterial ghosts, the surface properties of which resemble those of live bacteria, are highly immunogenic and are therefore strong adjuvants.
Despite considerable effort, oral immunization with encapsulated antigens is still limited by several issues that are specific to this route. The effectiveness of oral immunization has been established in several animal studies (mostly in mice), but clinical experience in this field has been mixed105,110,111,112. Specifically, serum antibody titres after oral delivery of liposome-encapsulated TT or DT to humans were variable and were lower than those observed for animals105. In another clinical study, oral immunization against enterotoxigenic E. coli using a microsphere-encapsulated colonization-factor antigen rendered protection against subsequent challenge in only 30% of patients110. In a more recent study, oral delivery of PLG-encapsulated CS6 antigen from E. coli generated antigen-secreting cells and an IgA response, but the differences between responses that were generated by encapsulated and non-encapsulated antigen were not great112. Attempting to scale up the results of oral immunization from animals to humans is generally problematic. Typically, the doses that are required to elicit an immune response through the oral route are substantially higher (by up to 100-fold) than those that are required when using injection113. This raises the crucial issue of the cost of immunization. Furthermore, oral immunization with non-living vaccines requires the use of carriers and adjuvants, and the safety of exposing the sensitive gastrointestinal tract to these compounds, in addition to the safety of exposure to the vaccine itself, remains to be carefully studied. A completely different solution to this issue has been offered by the use of genetically engineered plants as immunizing agents. This approach has yielded encouraging results in animals114 and humans115,116, but the safety of transgenic-plant vaccines needs to be further evaluated.
Nasal route. Intranasal delivery of vaccines using a nasal spray delivered into the nostrils is an attractive mode of immunization. The nose, similar to the mouth, is a practical site for vaccine administration, and nasopharynx-associated lymphoid tissue efficiently induces antigen-specific immune responses in both mucosal and systemic immune compartments117,118. A detailed review of nasal immunization can be found in Refs 118, 119. FluMist (MedImmune Vaccines, Inc.), a live influenza-virus vaccine, has already been approved by the Food and Drug Administration (United States) for intranasal administration. The development of non-living nasal vaccines has proved to be challenging, but considerable progress has been made in the past decade120. One nasal vaccine, an inactivated influenza-virus product known as Nasalflu (Berna Biotech AG), was introduced in Switzerland in 2000. However, it was withdrawn from the market in 2001 because of the vaccine-associated incidence of Bell's palsy, which was thought to originate from the use of E. coli LT as an adjuvant121. Efforts to develop other nasal vaccines, however, have continued to make progress. In the past decade, several clinical studies have confirmed the generation of local and systemic immunity after nasal immunization of humans against diphtheria and tetanus122, influenza123 and infection with Streptococcus mutans124. Varying local reactions, ranging from good tolerance to stinging, were reported in response to intranasal administration of vaccine122. A far larger number of studies in mice, pigs and monkeys also confirm the effectiveness of nasal immunization with a variety of vaccines119. Nasal vaccines have been delivered in various physical forms, including aerosolized liquids125, and liposome126 and microsphere formulations127, which can be administered together with various adjuvants88,128,129. Nasal immunization generally requires much lower antigen doses than does oral immunization, owing to lower enzymatic activity in the nasal cavity than in the gastrointestinal tract.
Vaginal or rectal route. Vaginal and rectal immunization through topical application of a cream has recently received attention for immunization against sexually transmitted diseases such as HIV/AIDS130. Vaginal immunization with a multicomponent peptide vaccine against HIV infection has been shown to induce local antibody responses in mice when administered together with strong adjuvants such as CTB131,132. DNA-vaccine strategies for preventing HIV infection using vaginal or rectal immunization have also been tested and have been shown to be effective at generating systemic and mucosal immune responses in mice133. Clinical experiments involving vaginal and rectal immunization against cholera, using a vaccine containing both whole cells and CTB, have yielded successful results134. In general, however, vaginal and rectal immunization with non-living vaccines has had limited success, owing to delivery and adjuvanticity issues.
Other routes. Additional mucosal routes, including pulmonary, ocular and sublingual administration of vaccines, have also been attempted in several cases. Aerosolized vaccines have been delivered through the pulmonary route, which aims to deliver vaccine at various levels of the bronchial tree, including the alveoli. Pulmonary delivery of vaccines, which targets bronchus-associated lymphoid tissue, has been effectively used for immunization of humans against measles, using a live attenuated virus135. Animal studies have also shown the effectiveness of non-living pulmonary vaccines, including inactivated influenza virus136.
Ocular immunization has been attempted against infection with herpes simplex virus (HSV). This was motivated by the strong need to generate ocular mucosal immunity to HSV, which commonly infects the eye in addition to other sites. Heat-killed HSV, as well as HSV-based subunit vaccines, generated effective mucosal immunity to HSV in pre-clinical animal studies that involved direct administration to the eye in the form of drops137,138. The effectiveness of sublingual immunization has also been shown in several studies in animals139,140. However, ocular and sublingual routes have been less well studied as generalized methods for immunization than have other mucosal routes.
A large body of literature has confirmed the merits of all modes of mucosal immunization. Products for mucosal immunization, especially nasal immunization, are under development in several companies. Immunization through mucosal routes (oral, ocular, pulmonary, nasal, vaginal or rectal) generates IgA-producing cells at the site of infection129. However, it should be noted that mucosal administration of antigen is not essential for the generation of antigen-specific IgA-producing plasma cells. Topical application of vaccine has also been shown to generate these cells and to induce mucosal immunity in the host19. Among the methods of mucosal immunization, nasal immunization seems to offer an optimal balance of immunogenicity, dosing and accessibility, as well as patient acceptability. Other mucosal routes are limited as generic modes of immunization. The oral route is limited by the difficulty in accessing M cells in the gastrointestinal tract and by dosing issues. The vaginal and rectal routes are limited by acceptability and immunogenicity issues. The issues of dosing and immunogenicity could be addressed through future research focused on developing strategies for better encapsulation, adjuvanticity and targeting. Fundamental studies focused on the transport of antigens or antigen carriers from the point of administration to the mucosal membrane will also bring new insights to mucosal immunization. These studies should be complemented by research focused on gaining a better understanding of the immunology of mucosal membranes, in particular by identification of specific target cells and receptors for vaccines and by study of the crosstalk between different mucosal compartments141.
Conclusions
The shortcomings of injections have led to active research and development of needle-free methods of immunization. The shift from needle-based to needle-free immunization is also catalysed, in part, by the realization that the skin and the mucosal membranes, which cannot be effectively accessed by conventional needles, are ideal targets for vaccine delivery12,142. Until recently, needle-free methods of immunization were restricted to liquid-jet injection and oral delivery of live attenuated pathogens. Considerable advances have been made in the past decade, especially in transdermal and nasal immunization, but it should be noted that most of the technologies that are discussed here are still at an early stage and lack detailed evaluation in terms of safety, toxicity, reproducibility and economic feasibility.
Cost of immunization is an important factor in the acceptability of new methods. According to the WHO, the current cost of administering three doses of the diphtheria–tetanus–pertussis vaccine is US$4–70 per child, depending on the country. Use of needle-free immunization might push this cost even higher, owing to the increased cost of development. The potential higher cost of needle-free immunization needs to be viewed in light of its benefits. Care needs to be taken when carrying out cost–benefit analysis of needle-free methods, because several benefits of needle-free methods are difficult to quantify. It is hoped that needle-free methods will lower the economic burden that is associated with needle-borne infections143 and will eventually prove to be economically feasible.
A comparison of the advantages and limitations of various methods of needle-free immunization (Table 1) makes it evident that there is no one method that is superior. Each method has advantages that are attractive for immunization. At the same time, all methods have limitations that need to be overcome. Each method is eventually likely to find its niche application, which will depend on the type of vaccine and the site of immunization, and on intellectual-property considerations. Opportunities in needle-free immunization have attracted an array of interdisciplinary researchers and businesses to the field of vaccine development. With the influx of new technologies and talent to this field, needle-free immunization is sure to become a reality.
References
Kermode, M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot. Int. 19, 95–103 (2004).
Nir, Y., Paz, A., Sabo, E. & Potasman, I. Fear of injections in young adults: prevalence and associations. Am. J. Trop. Med. Hyg. 68, 341–344 (2003).
Breau, L. M. et al. Facial expression of children receiving immunizations: a principal components analysis of the child facial coding system. Clin. J. Pain 17, 178–186 (2001).
Rosenstock, L. Needlestick injuries among healthcare workers. Centers for Disease Control and Prevention [online], <http://www.cdc.gov/washington/testimony/ps062200.htm> (2000).
World Health Organization. State of the World's Vaccines and Immunization (World Health Organization, Geneva, 1996).
Miller, M. A. & Pisani, E. The cost of unsafe injections. Bull. World Health Organ. 77, 808–811 (1999).
Kane, A. et al. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull. World Health Organ. 77, 801–807 (1999). This paper reports alarming estimates of the global and regional prevalence of HBV, HCV and HIV infections that might occur as a result of unsafe injection practices in developing countries. This underscores the motivation for developing needle-free methods of immunization.
World Health Organization. Safety of Injections: Global Facts & Figures (World Health Organization, Geneva, 2004).
Varmus, H. et al. Grand challenges in global health. Science 302, 398–399 (2003).
Levine, M. M. Can needle-free administration of vaccines become the norm in global immunization? Nature Med. 9, 99–103 (2003).
O'Hagan, D. T. & Rappuoli, R. Novel approaches to vaccine delivery. Pharm. Res. 21, 1519–1530 (2004).
Holmgren, J. & Czerkinsky, C. Mucosal immunity and vaccines. Nature Med. 11, S45–S53 (2005). This Review describes the properties of the mucosal immune system (that is, the mucosa-associated lymphoid tissue) and its mechanisms of antigen presentation. It also discusses advances in the development of mucosal vaccines and provides perspectives on future developments.
Weniger, B. G. Needle-free jet injection technology: bibliographic references, device & manufacturer roster, patents list and general/miscellaneous resources. Centers for Disease Control and Prevention [online], <http://www.cdc.gov/nip/dev/jetinject.htm#bibliography> (2005). This is an information portal about liquid-jet injectors. A comprehensive list of past and current manufacturers of liquid-jet injectors and of published literature is provided.
Hingson, R. A. & Figge, F. H. J. A survey of the development of jet injection in parenteral therapy. Curr. Res. Anesth. Analg. 31, 361–366 (1952).
Peachman, K. K., Rao, M. & Alving, C. R. Immunization with DNA through the skin. Methods 31, 232–242 (2003).
Babiuk, S. et al. Cutaneous vaccination: the skin as an immunologically active tissue and the challenge of antigen delivery. J. Control. Release 66, 199–214 (2000).
Bodey, B., Bodey, B. Jr & Kaiser, H. E. Dendritic type, accessory cells within the mammalian thymic microenvironment. Antigen presentation in the dendritic neuro-endocrine–immune cellular network. In Vivo 11, 351–370 (1997).
Stoitzner, P. et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J. Invest. Dermatol. 120, 266–274 (2003).
Gockel, C. M., Bao, S. & Beagley, K. W. Transcutaneous immunization induces mucosal and systemic immunity: a potent method for targeting immunity to the female reproductive tract. Mol. Immunol. 37, 537–544 (2000).
Glenn, G. M. et al. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J. Immunol. 161, 3211–3214 (1998).
Kenney, R. T. et al. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 351, 2295–2301 (2004).
Ren, S. et al. Low-volume jet injection for intradermal immunization in rabbits. BMC Biotechnol. [online] 2, 10 (2002).
Baxter, J. in Fundamental Mechanisms of Drug Delivery by Jet Injection: Basis for the Development of a Painless Microjet Injector. 161 Thesis, Univ. California, Santa Barbara (2004).
Weniger, B. G. Jet injection of vaccines: overview and challenges for mass vaccination with jet injections (JIs). United States Department of Health and Human Services [online], <http://www.hhs.gov/nvpo/meetings/dec2003/Contents/ThursdayPM/Weniger.pdf> (2003).
Williams, J. et al. Hepatitis A vaccine administration: comparison between jet-injector and needle injection. Vaccine 18, 1939–1943 (2000).
Jackson, L. A. et al. Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle-free jet injectors. Vaccine 19, 4703–4709 (2001).
Mathei, C., Van Damme, P. & Meheus, A. Hepatitis B vaccine administration: comparison between jet-gun and syringe and needle. Vaccine 15, 402–404 (1997).
Canter, J. et al. An outbreak of hepatitis-B associated with jet injections in a weight-reduction clinic. Arch. Intern. Med. 150, 1923–1927 (1990).
Hoffman, P. N. et al. A model to assess the infection potential of jet injectors used in mass immunisation. Vaccine 19, 4020–4027 (2001).
Weniger, B. G. New high-speed jet injectors for mass vaccination: pros and cons of disposable-cartridge jet injectors (DCJIs) versus multi-use-nozzle jet injectors (MUNJIs). World Health Organization [online], <http://www.who.int/vaccine_research/about/gvrf_2004/en/ gvrf_2004_weniger.pdf> (2004).
Dimache, G. et al. A clinical, epidemiological and laboratory study on avoiding the risk of transmitting viral hepatitis during vaccinations with the Dermojet protected by an anticontaminant disposable device. Vaccine 15, 1010–1013 (1997).
Cooper, J. A., Bromley, L. M., Baranowski, A. P. & Barker, S. G. Evaluation of a needle-free injection system for local anaesthesia prior to venous cannulation. Anaesthesia 55, 247–250 (2000).
Epstein, J. E. et al. Safety, tolerability, and lack of antibody responses after administration of a PfCSP DNA malaria vaccine via needle or needle-free jet injection, and comparison of intramuscular and combination intramuscular/intradermal routes. Hum. Gene Ther. 13, 1551–1560 (2002).
Haensler, J. et al. Intradermal DNA immunization by using jet-injectors in mice and monkeys. Vaccine 17, 628–638 (1999).
Raviprakash, K. et al. Needle-free Biojector injection of a dengue virus type 1 DNA vaccine with human immunostimulatory sequences and the GM-CSF gene increases immunogenicity and protection from virus challenge in Aotus monkeys. Virology 315, 345–352 (2003).
Schramm, J. & Mitragotri, S. Transdermal drug delivery by jet injectors: energetics of jet formation and penetration. Pharm. Res. 19, 1673–1679 (2002).
Schramm-Baxter, J. & Mitragotri, S. Needle-free jet injections: dependence of jet penetration and dispersion in the skin on jet power. J. Control. Release 97, 527–535 (2004).
Kendall, M., Mitchell, T. & Wrighton-Smith, P. Intradermal ballistic delivery of micro-particles into excised human skin for pharmaceutical applications. J. Biomech. 37, 1733–1741 (2004).
Chen, D., Maa, Y. F. & Haynes, J. R. Needle-free epidermal powder immunization. Expert Rev. Vaccines 1, 265–276 (2002).
Chen, D. et al. Epidermal powder immunization of mice and monkeys with an influenza vaccine. Vaccine 21, 2830–2836 (2003).
Chen, D. et al. Serum and mucosal immune responses to an inactivated influenza virus vaccine induced by epidermal powder immunization. J. Virol. 75, 7956–7965 (2001).
Chen, D. et al. Adjuvantation of epidermal powder immunization. Vaccine 19, 2908–2917 (2001).
Chen, D. et al. Epidermal powder immunization using non-toxic bacterial enterotoxin adjuvants with influenza vaccine augments protective immunity. Vaccine 20, 2671–2679 (2002).
Dean, H. J., Haynes, J. & Schmaljohn, C. The role of particle-mediated DNA vaccines in biodefense preparedness. Adv. Drug Deliv. Rev. 57, 1315–1342 (2005). This is a review of recent developments in particle-mediated DNA vaccines. An overview of the principles and applications of EPI with respect to DNA vaccination is provided.
Kim, T. W. et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J. Clin. Invest. 112, 109–117 (2003).
Dean, H. J. & Chen, D. Epidermal powder immunization against influenza. Vaccine 23, 681–686 (2004).
Roy, M. J. et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 19, 764–778 (2000).
Tacket, C. O. et al. Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine 17, 2826–2829 (1999).
Roberts, L. K. et al. Clinical safety and efficacy of a powdered hepatitis B nucleic acid vaccine delivered to the epidermis by a commercial prototype device. Vaccine 23, 4867–4878 (2005).
Kendall, M., Rishworth, S., Carter, F. & Mitchell, T. Effects of relative humidity and ambient temperature on the ballistic delivery of micro-particles to excised porcine skin. J. Invest. Dermatol. 122, 739–746 (2004).
Prausnitz, M. R., Mitragotri, S. &. Langer, R. Current status and future potential of transdermal drug delivery. Nature Rev. Drug Discov. 3, 115–124 (2004). This paper reviews transdermal drug delivery. It describes the principles of dermal penetration, its current status and the methods that are under development to increase the permeability of the skin to molecules, including vaccines. These methods have an important role in immunization by topical application.
Barquet, N. & Domingo, P. Smallpox: the triumph over the most terrible of the ministers of death. Ann. Intern. Med. 127, 635–642 (1997).
Fan, H., Lin, Q., Morrissey, G. R. & Khavari, P. A. Immunization via hair follicles by topical application of naked DNA to normal skin. Nature Biotechnol. 17, 870–872 (1999).
Bouwstra, J. et al. New aspects of the skin barrier organization. Skin Pharmacol. Appl. Skin Physiol. 14 (Suppl. 1), 52–62 (2001).
Glenn, G. M., Rao, M., Matyas, G. R. & Alving, C. R. Skin immunization made possible by cholera toxin. Nature 391, 851 (1998).
Glenn, G. M. et al. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev. Vaccines 2, 253–267 (2003).
Hammond, S. A., Walwender, D., Alving, C. R. & Glenn, G. M. Transcutaneous immunization: T cell responses and boosting of existing immunity. Vaccine 19, 2701–2707 (2001).
Belyakov, I. M. et al. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J. Clin. Invest. 113, 998–1007 (2004).
Kenney, R. T. et al. Induction of protective immunity against lethal anthrax challenge with a patch. J. Infect. Dis. 190, 774–782 (2004).
Frech, S. A. et al. Improved immune responses to influenza vaccination in the elderly using an immunostimulant patch. Vaccine 23, 946–950 (2005).
Scharton-Kersten, T. et al. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins, subunits, and unrelated adjuvants. Infect. Immun. 68, 5306–5313 (2000).
Scharton-Kersten, T. et al. Principles of transcutaneous immunization using cholera toxin as an adjuvant. Vaccine 17, S37–S43 (1999).
Guerena-Burgueno, F. et al. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 70, 1874–1880 (2002).
Glenn, G. M. et al. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nature Med. 6, 1403–1406 (2000). This is one of the pioneering reports of immunization by topical application of vaccines. It describes a clinical study of adjuvant-patch-mediated cutaneous immunization.
Gupta, P. N. et al. Tetanus toxoid-loaded transfersomes for topical immunization. J. Pharm. Pharmacol. 57, 295–301 (2005).
Baca-Estrada, M. E., Foldvari, M., Babiuk, S. L. & Babiuk, L. A. Vaccine delivery: lipid-based delivery systems. J. Biotechnol. 83, 91–104 (2000).
Cui, Z. et al. Novel ethanol-in-fluorocarbon microemulsions for topical genetic immunization. Pharm. Res. 20, 16–23 (2003).
Cui, Z. & Mumper, R. J. Topical immunization using nanoengineered genetic vaccines. J. Control. Release 81, 173–184 (2002).
Cui, Z. & Mumper, R. J. Chitosan-based nanoparticles for topical genetic immunization. J. Control. Release 75, 409–419 (2001).
Bramson, J. et al. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 10, 251–260 (2003).
Matriano, J. A. et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm. Res. 19, 63–70 (2002).
Prausnitz, M. R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 56, 581–587 (2004).
Mikszta, J. A. et al. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nature Med. 8, 415–419 (2002).
Kahlon, R. et al. Optimization of epicutaneous immunization for the induction of CTL. Vaccine 21, 2890–2899 (2003).
Takigawa, M. et al. Percutaneous peptide immunization via corneum barrier-disrupted murine skin for experimental tumor immunoprophylaxis. Ann. NY Acad. Sci. 941, 139–146 (2001).
Seo, N. et al. Percutaneous peptide immunization via corneum barrier-disrupted murine skin for experimental tumor immunoprophylaxis. Proc. Natl Acad. Sci. USA 97, 371–376 (2000).
Van Kampen, K. R. et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 23, 1029–1036 (2005).
Tezel, A., Paliwal, S., Shen, Z. & Mitragotri, S. Low-frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine 23, 3800–3807 (2005).
Tezel, A. & Mitragotri, S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys. J. 85, 3502–3512 (2003).
Zhang, L., Nolan, E., Kreitschitz, S. & Rabussay, D. P. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim. Biophys. Acta 1572, 1–9 (2002).
Misra, A., Ganga, S. & Upadhyay, P. Needle-free, non-adjuvanted skin immunization by electroporation-enhanced transdermal delivery of diphtheria toxoid and a candidate peptide vaccine against hepatitis B virus. Vaccine 18, 517–523 (1999).
Archer, D. F. et al. Assessment of compliance with a weekly contraceptive patch (Ortho Evra/Evra) among North American women. Fertil. Steril. 77, S27–S31 (2002).
Yuki, Y. & Kiyono, H. New generation of mucosal adjuvants for the induction of protective immunity. Rev. Med. Virol. 13, 293–310 (2003).
Fooks, A. R. Development of oral vaccines for human use. Curr. Opin. Mol. Ther. 2, 80–86 (2000).
Brown, W. R. Enteric immunization: promises and challenges. Dig. Dis. 14, 192–200 (1996).
Lambkin, I. & Pinilla, C. Targeting approaches to oral drug delivery. Expert Opin. Biol. Ther. 2, 67–73 (2002).
Kersten, G. & Hirschberg, H. Antigen delivery systems. Expert Rev. Vaccines 3, 453–462 (2004).
Freytag, L. C. & Clements, J. D. Mucosal adjuvants. Vaccine 23, 1804–1813 (2005).
Eriksson, K. & Holmgren, J. Recent advances in mucosal vaccines and adjuvants. Curr. Opin. Immunol. 14, 666–672 (2002).
Pizza, M. et al. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19, 2534–2541 (2001).
Nellore, R. V., Pande, P. G., Young, D. & Bhagat, H. R. Evaluation of biodegradable microspheres as vaccine adjuvant for hepatitis B surface antigen. J. Parenter. Sci. Technol. 46, 176–180 (1992).
Esparza, I. & Kissel, T. Parameters affecting the immunogenicity of microencapsulated tetanus toxoid. Vaccine 10, 714–720 (1992).
Challacombe, S. J. et al. Enhanced secretory IgA and systemic IgG antibody responses after oral immunization with biodegradable microparticles containing antigen. Immunology 76, 164–168 (1992).
Stertman, L., Strindeliu, L. & Sjoholm, I. Starch microparticles as an adjuvant in immunisation: effect of route of administration on the immune response in mice. Vaccine 22, 2863–2872 (2004).
Ren, J. M. et al. PELA microspheres loaded H. pylori lysates and their mucosal immune response. World J. Gastroenterol. 8, 1098–1102 (2002).
Wikingsson, L. & Sjoholm, I. Polyacryl starch microparticles as adjuvant in oral immunisation, inducing mucosal and systemic immune responses in mice. Vaccine 20, 3355–3363 (2002).
Preis, I. & Langer, R. S. A single-step immunization by sustained antigen release. J. Immunol. Methods 28, 193–197 (1979).
Langer, R., Cleland, J. L. & Hanes, J. New advances in microsphere-based single-dose vaccines. Adv. Drug Deliv. Rev. 28, 97–119 (1997).
Jones, D. H. et al. Poly(DL-lactide-co-glycolide)-encapsulated plasmid DNA elicits systemic and mucosal antibody responses to encoded protein after oral administration. Vaccine 15, 814–817 (1997).
Chew, J. L. et al. Chitosan nanoparticles containing plasmid DNA encoding house dust mite allergen, Der p 1 for oral vaccination in mice. Vaccine 21, 2720–2729 (2003).
Roy, K., Mao, H. Q., Huang, S. K. & Leong, K. W. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nature Med. 5, 387–391 (1999).
Ermak, T. H. & Giannasca, P. J. Microparticle targeting to M cells. Adv. Drug Deliv. Rev. 34, 261–283 (1998).
Perrie, Y., Obrenovic, M., McCarthy, D. & Gregoriadis, G. Liposome (Lipodine)-mediated DNA vaccination by the oral route. J. Liposome Res. 12, 185–197 (2002).
Chen, H. & Langer, R. Oral particulate delivery: status and future trends. Adv. Drug Deliv. Rev. 34, 339–350 (1998).
Mirchamsy, H. et al. Stimulating role of toxoids-laden liposomes in oral immunization against diphtheria and tetanus infections. Biologicals 24, 343–350 (1996).
Chen, H., Torchilin, V. & Langer, R. Lectin-bearing polymerized liposomes as potential oral vaccine carriers. Pharm. Res. 13, 1378–1383 (1996).
Kersten, G. F. & Crommelin, D. J. Liposomes and ISCOMs. Vaccine 21, 915–920 (2003).
Patel, G. B., Omri, A., Deschatelets, L. & Sprott, G. D. Safety of archaeosome adjuvants evaluated in a mouse model. J. Liposome Res. 12, 353–372 (2002).
Jalava, K., Eko, F. O., Riedmann, E. & Lubitz, W. Bacterial ghosts as carrier and targeting systems for mucosal antigen delivery. Expert Rev. Vaccines 2, 45–51 (2003).
Tacket, C. O. et al. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine 12, 1270–1274 (1994).
Lambert, J. S. et al. A Phase I safety and immunogenicity trial of UBI microparticulate monovalent HIV-1 MN oral peptide immunogen with parenteral boost in HIV-1 seronegative human subjects. Vaccine 19, 3033–3042 (2001).
Katz, D. E. et al. Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli (ETEC) CS6 antigen. Vaccine 21, 341–346 (2003).
Brayden, D. J. Oral vaccination in man using antigens in particles: current status. Eur. J. Pharm. Sci. 14, 183–189 (2001). This paper provides a critical review of the field of oral vaccine delivery. It discusses issues that are associated with the scaling up of animal results to humans.
Haq, T. A., Mason, H. S., Clements, J. D. & Arntzen, C. J. Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science 268, 714–716 (1995).
Tacket, C. O. et al. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nature Med. 4, 607–609 (1998). This paper reports on studies that involved the immunization of humans with transgenic vegetables. Potatoes expressing LT were used to immunize human volunteers, and the generation of neutralizing antibodies was confirmed.
Thanavala, Y. et al. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl Acad. Sci. USA 102, 3378–3382 (2005).
Kiyono, H. & Fukuyama, S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nature Rev. Immunol. 4, 699–710 (2004).
Vajdy, M. & O'Hagan, D. T. Microparticles for intranasal immunization. Adv. Drug Deliv. Rev. 51, 127–141 (2001).
Davis, S. S. Nasal vaccines. Adv. Drug Deliv. Rev. 51, 21–42 (2001). This paper provides an overview of nasal immunization. The structure and function of nasopharynx-associated lymphoid tissue and its role in nasal immunization are discussed.
Haneberg, B. & Holst, J. Can nonliving nasal vaccines be made to work? Expert Rev. Vaccines 1, 227–232 (2002).
Mutsch, M. et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350, 896–903 (2004).
Aggerbeck, H., Gizurarson, S., Wantzin, J. & Heron, I. Intranasal booster vaccination against diphtheria and tetanus in man. Vaccine 15, 307–316 (1997).
Gluck, U., Gebbers, J. O. & Gluck, R. Phase 1 evaluation of intranasal virosomal influenza vaccine with and without Escherichia coli heat-labile toxin in adult volunteers. J. Virol. 73, 7780–7786 (1999).
Li, F. et al. Intranasal immunization of humans with Streptococcus mutans antigens. Oral Microbiol. Immunol. 18, 271–277 (2003).
Roth, Y., Chapnik, J. S. & Cole, P. Feasibility of aerosol vaccination in humans. Ann. Otol. Rhinol. Laryngol. 112, 264–270 (2003).
de Jonge, M. I. et al. Intranasal immunisation of mice with liposomes containing recombinant meningococcal OpaB and OpaJ proteins. Vaccine 22, 4021–4028 (2004).
Alpar, H. O., Somavarapu, S., Atuah, K. N. & Bramwell, V. W. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 57, 411–430 (2005).
Singh, M. & O'Hagan, D. T. Recent advances in vaccine adjuvants. Pharm. Res. 19, 715–728 (2002).
Vajdy, M. et al. Mucosal adjuvants and delivery systems for protein-, DNA- and RNA-based vaccines. Immunol. Cell Biol. 82, 617–627 (2004).
Stevceva, L. & Strober, W. Mucosal HIV vaccines: where are we now? Curr. HIV Res. 2, 1–10 (2004).
Russell, M. W. Immunization for protection of the reproductive tract: a review. Am. J. Reprod. Immunol. 47, 265–268 (2002).
Kato, H. et al. Rectal and vaginal immunization with a macromolecular multicomponent peptide vaccine candidate for HIV-1 infection induces HIV-specific protective immune responses. Vaccine 18, 1151–1160 (2000).
Hamajima, K. et al. Systemic and mucosal immune responses in mice after rectal and vaginal immunization with HIV-DNA vaccine. Clin. Immunol. 102, 12–18 (2002).
Wassen, L. et al. Local intravaginal vaccination of the female genital tract. Scand. J. Immunol. 44, 408–414 (1996).
Dilraj, A. et al. Response to different measles vaccine strains given by aerosol and subcutaneous routes to schoolchildren: a randomised trial. Lancet 355, 798–803 (2000).
Smith, D. J., Bot, S., Dellamary, L. & Bot, A. Evaluation of novel aerosol formulations designed for mucosal vaccination against influenza virus. Vaccine 21, 2805–2812 (2003).
Narang, H. K. Efficacy of herpes vaccine and acyclovir (ACV) in a rabbit model following intraocular inoculation of herpes simplex virus. J. Chemother. 7, 210–215 (1995).
Nesburn, A. B. et al. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology 252, 200–209 (1998).
BenMohamed, L. et al. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur. J. Immunol. 32, 2274–2281 (2002).
Montgomery, P. C. & Rafferty, D. E. Induction of secretory and serum antibody responses following oral administration of antigen with bioadhesive degradable starch microparticles. Oral Microbiol. Immunol. 13, 139–149 (1998).
Bouvet, J. P., Decroix, N. & Pamonsinlapatham, P. Stimulation of local antibody production: parenteral or mucosal vaccination? Trends Immunol. 23, 209–213 (2002).
Kupper, T. S. & Fuhlbrigge, R. C. Immune surveillance in the skin: mechanisms and clinical consequences. Nature Rev. Immunol. 4, 211–222 (2004). This is a review of the immune function of the skin, which has an important role in immunization by topical application. Interactions between the innate and adaptive immune systems in the skin and their role in immune surveillance are discussed.
Ekwueme, D. U., Weniger, B. G. & Chen, R. T. Model-based estimates of risks of disease transmission and economic costs of seven injection devices in sub-Saharan Africa. Bull. World Health Organ. 80, 859–870 (2002).
Alexander, L. N. et al. Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA 292, 1696–1701 (2004).
Centers for Disease Control and Prevention. Suspension of rotavirus vaccine after reports of intussusception — United States, 1999. MMWR Morb. Mortal. Wkly Rep. 53, 786–789 (2004).
O'Hagan, D. T. & Rappuoli, R. The safety of vaccines. Drug Discov. Today 9, 846–854 (2004).
O'Hagan, D. T. Recent developments in vaccine delivery systems. Curr. Drug Targets Infect. Disord. 1, 273–286 (2001).
Bourlais, C. L. et al. Ophthalmic drug delivery systems — recent advances. Prog. Retin. Eye Res. 17, 33–58 (1998).
Davis, J. L., Gilger, B. C. & Robinson, M. R. Novel approaches to ocular drug delivery. Curr. Opin. Mol. Ther. 6, 195–205 (2004).
Burkoth, T. L. et al. Transdermal and transmucosal powdered drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 16, 331–384 (1999).
Vidgren, M. T. & Kublik, H. Nasal delivery systems and their effect on deposition and absorption. Adv. Drug Deliv. Rev. 29, 157–177 (1998).
Hussain, A. A. Intranasal drug delivery. Adv. Drug Deliv. Rev. 29, 39–49 (1998).
Edwards, D. A. & Dunbar, C. Bioengineering of therapeutic aerosols. Annu. Rev. Biomed. Eng. 4, 93–107 (2002).
Sastry, S. V., Nyshadham, J. R. & Fix, J. A. Recent technological advances in oral drug delivery — a review. Pharm. Sci. Technol. Today 3, 138–145 (2000).
Hussain, A. & Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release 103, 301–313 (2005).
Mitragotri, S. & Kost, J. Low-frequency sonophoresis: a review. Adv. Drug Deliv. Rev. 56, 589–601 (2004).
Denet, A. R., Vanbever, R. & Preat, V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 56, 659–674 (2004).
Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 56, 675–711 (2004).
Acknowledgements
The author acknowledges support from The Whitaker Foundation (United States) and the National Institutes of Health (United States). The author thanks P. Karande, J. Champion, A. Jain and S. Paliwal for assistance during manuscript preparation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Samir Mitragotri holds shares in Sontra Medical Corporation (United States).
Related links
Related links
DATABASES
Infectious disease information
FURTHER INFORMATION
Glossary
- LIVE ATTENUATED VIRUS
-
A weakened mutant of a wild-type virus that is antigenic but not infectious.
- LANGERHANS CELL
-
A type of dendritic cell (which are professional antigen-presenting cells) that is localized in the epidermal layer of the skin.
- SEROCONVERSION
-
Development of a detectable concentration of pathogen-specific antibodies in the serum as a result of infection or immunization.
- ADJUVANT
-
An agent that is mixed with an antigen and increases the immune response to that antigen following immunization.
- COLLOIDAL CARRIER
-
A stable system of small particles of lipids, polymers or any other material that encapsulate a vaccine.
- MICROEMULSION
-
A stabilized emulsion (that is, a preparation of two immiscible liquids, in which one is dispersed in the other) in which the dispersed droplets are extremely small.
- MICROFOLD CELL
-
(M cell). A specialized type of epithelial cell that delivers antigens from the lumen directly to intraepithelial lymphocytes and to subepithelial lymphoid tissues, using transepithelial vesicular transport.
- PEYER'S PATCH
-
A section of the intestinal epithelium that contains microfold cells. These regions form the mucosa-associated lymphoid tissue.
- LIPOSOME
-
A lipid vesicle that encapsulates vaccines in a lipid-bilayer membrane and facilitates their delivery.
- BACTERIAL GHOST
-
A bacterium, the cytoplasmic contents of which have been removed.
Rights and permissions
About this article
Cite this article
Mitragotri, S. Immunization without needles. Nat Rev Immunol 5, 905–916 (2005). https://doi.org/10.1038/nri1728
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1728
This article is cited by
-
Experimental investigation on penetration performance of larger volume needle-free injection device
Journal of Mechanical Science and Technology (2020)
-
C-di-GMP with influenza vaccine showed enhanced and shifted immune responses in microneedle vaccination in the skin
Drug Delivery and Translational Research (2020)
-
An experimental study of a spring-loaded needle-free injector: Influence of the ejection volume and injector orifice diameter
Journal of Mechanical Science and Technology (2019)
-
Investigations into insertion force of electrochemically micro-textured hypodermic needles
The International Journal of Advanced Manufacturing Technology (2019)