Key Points
-
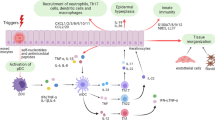
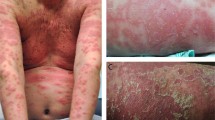
Psoriasis is an inflammatory disease of the skin that is mediated by T cells, dendritic cells (DCs), and a broad range of cytokines and chemokines. However, much of the clinical disease phenotype is caused by hyperplasia of epidermal keratinocytes, altered differentiation of these cells in a 'regenerative' pathway and increased growth of dermal blood vessels (causing thick, red, scaly plaques on the skin surface).
-
Psoriasis has been proposed to be a T-cell-mediated autoimmune disease, because clonal populations of T cells have been identified in skin lesions and the overall characteristics of these T cells indicate that they are T helper 1 cells and type 1 cytotoxic T-cell effectors. However, as reactivity to a self-antigen has not been shown, others have suggested that immune activation in the skin is triggered through innate immune pathways stimulated by Toll-like receptors, heat-shock-protein receptors or glycolipid-antigen receptors.
-
The molecules that are involved in pathogenic inflammation in the skin have been defined by large-scale gene-expression studies, and many interactive pathways of inflammation have been suggested to occur. Two ideas that stem from this work are that, first, lymphoid tissue (self-perpetuating infiltrates of T cells and DCs) is organized in the skin by local expression of chemokines and that, second, a 'type 1' pathway of inflammatory gene products (involving interleukin-23, interferon-γ and signal transducer and activator of transcription 1) is central to pathogenic inflammation in psoriasis.
-
There is a strong interrelationship between regenerative growth of the epidermis (as programmed for wound healing) and activation of cellular immunity through innate and adaptive immune pathways. Genes that confer susceptibility to psoriasis could alter keratinocyte differentiation responses, cellular immune responses or common molecular pathways that control the balance of epithelial-cell hyperplasia and immune activation in the skin.
-
Approximately half of all patients with psoriasis have a susceptibility variant from the MHC class I region. However, some patients can develop psoriasis without any contribution from the MHC locus. In most cases, several genetic variants are required for disease to develop. Genetic studies of families have localized 20 such loci.
-
So far, only a handful of psoriasis-susceptibility genes have been identified in a single population. Their role in disease pathogenesis and the effect of environmental triggers on these genes is not clear.
-
One susceptibility variant for psoriasis that maps to human chromosome 17q25 has been shown to result in the loss of an enhancer binding site for the RUNX (runt-related transcription factor) family of transcription factors. Changes in RUNX-binding sites have also been seen at different loci in individuals with either systemic lupus erythematosus or rheumatoid arthritis, indicating that some changes in psoriasis are within pathways that are altered in other autoimmune diseases.
-
Several variants for psoriasis and other autoimmune or inflammatory diseases reduce the threshold for T-cell activation or affect immunological synapse formation or dissolution.
Abstract
Psoriasis is a chronic inflammatory disorder of the skin that is mediated by T cells, dendritic cells and inflammatory cytokines. We now understand many of the cellular alterations that underlie this disease, and genomic approaches have recently been used to assess the alterations of gene expression in psoriatic skin lesions. Genetic susceptibility factors that contribute to predisposition to psoriasis are now also being identified. It is hoped that we will soon be able to correlate the cellular pathogenesis that occurs in psoriasis with these genetic factors. In this Review article, we describe what is known about genes that confer increased susceptibility to psoriasis, and we integrate this with what is known about the molecular and cellular mechanisms that occur in other inflammatory and autoimmune disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lebwohl, M. Psoriasis. Lancet 361, 1197–1204 (2003).
Nickoloff, B. J., Bonish, B., Huang, B. B. & Porcelli, S. A. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J. Dermatol. Sci. 24, 212–225 (2000).
Chang, J. C. et al. CD8+ T cells in psoriatic lesions preferentially use T-cell receptor Vβ3 and/or Vβ13.1 genes. Proc. Natl Acad. Sci. USA 91, 9282–9286 (1994).
Prinz, J. C. et al. Selection of conserved TCR VDJ rearrangements in chronic psoriatic plaques indicates a common antigen in psoriasis vulgaris. Eur. J. Immunol. 29, 3360–3368 (1999).
Vollmer, S., Menssen, A. & Prinz, J. C. Dominant lesional T cell receptor rearrangements persist in relapsing psoriasis but are absent from nonlesional skin: evidence for a stable antigen-specific pathogenic T cell response in psoriasis vulgaris. J. Invest. Dermatol. 117, 1296–1301 (2001).
Bos, J. D., Hulsebosch, H. J., Krieg, S. R., Bakker, P. M. & Cormane, R. H. Immunocompetent cells in psoriasis. In situ immunophenotyping by monoclonal antibodies. Arch. Dermatol. Res. 275, 181–189 (1983).
Ellis, C. N. et al. Cyclosporine improves psoriasis in a double-blind study. JAMA 256, 3110–3116 (1986).
Baker, B. S. et al. The effects of cyclosporin A on T lymphocyte and dendritic cell sub-populations in psoriasis. Br. J. Dermatol. 116, 503–510 (1987).
Tigalonowa, M., Bjerke, J. R., Gallati, H. & Matre, R. Immunological changes following treatment of psoriasis with cyclosporin. Acta Derm. Venereol. Suppl. (Stockh.) 146, 142–145 (1989).
Jegasothy, B. V. et al. Tacrolimus (FK 506) — a new therapeutic agent for severe recalcitrant psoriasis. Arch. Dermatol. 128, 781–785 (1992).
Weinshenker, B. G., Bass, B. H., Ebers, G. C. & Rice, G. P. Remission of psoriatic lesions with muromonab-CD3 (orthoclone OKT3) treatment. J. Am. Acad. Dermatol. 20, 1132–1133 (1989).
Prinz, J. et al. Chimaeric CD4 monoclonal antibody in treatment of generalised pustular psoriasis. Lancet 338, 320–321 (1991).
Gottlieb, S. L. et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nature Med. 1, 442–447 (1995). This study provided the first evidence that disease remission could be achieved by highly selective targeting of activated T cells. The subsequent testing and development of T-cell-targeted biological drugs for the treatment of psoriasis were stimulated by this work.
Abrams, J. R. et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J. Clin. Invest. 103, 1243–1252 (1999).
Abrams, J. R. et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4–immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J. Exp. Med. 192, 681–694 (2000).
Krueger, J. G. The immunologic basis for the treatment of psoriasis with new biologic agents. J. Am. Acad. Dermatol. 46, 1–23 (2002).
Gottlieb, A. B. Psoriasis: emerging therapeutic strategies. Nature Rev. Drug Discov. 4, 19–34 (2005). References 16 and 17 introduce more than 20 immune-targeted biological agents that have been tested as potential therapeutics for psoriasis vulgaris.
Snowden, J. A. & Heaton, D. C. Development of psoriasis after syngeneic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br. J. Dermatol. 137, 130–132 (1997).
Gardembas-Pain, M. et al. Psoriasis after allogeneic bone marrow transplantation. Arch. Dermatol. 126, 1523 (1990).
Daikeler, T., Gunaydin, I., Einsele, H., Kanz, L. & Kotter, I. Transmission of psoriatic arthritis by allogeneic bone marrow transplantation for chronic myelogenous leukaemia from an HLA-identical donor. Rheumatology (Oxford) 38, 89–90 (1999).
Wrone-Smith, T. & Nickoloff, B. J. Dermal injection of immunocytes induces psoriasis. J. Clin. Invest. 98, 1878–1887 (1996). This paper describes the first xenotransplantation model in which psoriasis was induced.
Boyman, O. et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199, 731–736 (2004). In this study, uninvolved skin grafted onto mice spontaneously becomes psoriatic, in contrast to the psoriasis that is induced in other xenotransplantation models (which require co-transfer of immune cells).
Banno, T., Adachi, M., Mukkamala, L. & Blumenberg, M. Unique keratinocyte-specific effects of interferon-γ that protect skin from viruses, identified using transcriptional profiling. Antivir. Ther. 8, 541–554 (2003).
Banno, T., Gazel, A. & Blumenberg, M. Effects of tumor necrosis factor-α (TNFα) in epidermal keratinocytes revealed using global transcriptional profiling. J. Biol. Chem. 279, 32633–32642 (2004).
Barisic-Drusko, V. & Rucevic, I. Trigger factors in childhood psoriasis and vitiligo. Coll. Antropol. 28, 277–285 (2004).
Quesada, J. R. & Gutterman, J. U. Psoriasis and α-interferon. Lancet 1, 1466–1468 (1986).
Gilliet, M. et al. Psoriasis triggered by Toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch. Dermatol. 140, 1490–1495 (2004).
Ragaz, A. & Ackerman, A. B. Evolution, maturation, and regression of lesions of psoriasis. New observations and correlation of clinical and histologic findings. Am. J. Dermatopathol. 1, 199–214 (1979).
Homey, B. et al. CCL27–CCR10 interactions regulate T cell-mediated skin inflammation. Nature Med. 8, 157–165 (2002).
Zhou, X. et al. Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol. Genomics 13, 69–78 (2003). This reference, together with reference 33, describes the pathogenesis of psoriasis from the genomic view. It provides the basis for the view that psoriasis is mediated by a 'type 1' pathway of inflammation and that psoriatic lesions contain organized lymphoid tissue.
Chamian, F. et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc. Natl Acad. Sci. USA 102, 2075–2080 (2005).
Kurth, I. et al. Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J. Exp. Med. 194, 855–861 (2001).
Lew, W., Bowcock, A. M. & Krueger, J. G. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and 'type 1' inflammatory gene expression. Trends Immunol. 25, 295–305 (2004).
Rottman, J. B., Smith, T. L., Ganley, K. G., Kikuchi, T. & Krueger, J. G. Potential role of the chemokine receptors CXCR3, CCR4, and the integrin αEβ7 in the pathogenesis of psoriasis vulgaris. Lab. Invest. 81, 335–347 (2001).
Ferenczi, K., Burack, L., Pope, M., Krueger, J. G. & Austin, L. M. CD69, HLA-DR and the IL-2R identify persistently activated T cells in psoriasis vulgaris lesional skin: blood and skin comparisons by flow cytometry. J. Autoimmun. 14, 63–78 (2000).
Austin, L. M., Ozawa, M., Kikuchi, T., Walters, I. B. & Krueger, J. G. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-γ, interleukin-2, and tumor necrosis factor-α, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Invest. Dermatol. 113, 752–759 (1999).
Austin, L. M., Coven, T. R., Bhardwaj, N., Steinman, R. & Krueger, J. G. Intraepidermal lymphocytes in psoriatic lesions are activated GMP-17(TIA-1)+CD8+CD3+ CTLs as determined by phenotypic analysis. J. Cutan. Pathol. 25, 79–88 (1998).
Yawalkar, N., Schmid, S., Braathen, L. R. & Pichler, W. J. Perforin and granzyme B may contribute to skin inflammation in atopic dermatitis and psoriasis. Br. J. Dermatol. 144, 1133–1139 (2001).
Aggarwal, S., Ghilardi, N., Xie, M. H., de Sauvage, F. J. & Gurney, A. L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 (2003).
Teunissen, M. B., Koomen, C. W., de Waal Malefyt, R., Wierenga, E. A. & Bos, J. D. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Invest. Dermatol. 111, 645–649 (1998).
Lee, E. et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 199, 125–130 (2004).
Nickoloff, B. J., Wrone-Smith, T., Bonish, B. & Porcelli, S. A. Response of murine and normal human skin to injection of allogeneic blood-derived psoriatic immunocytes: detection of T cells expressing receptors typically present on natural killer cells, including CD94, CD158, and CD161. Arch. Dermatol. 135, 546–552 (1999).
Martin, M. P. et al. Susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. J. Immunol. 169, 2818–2822 (2002).
Sugiyama, H. et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 174, 164–173 (2005).
Cerio, R., Griffiths, C. E., Cooper, K. D., Nickoloff, B. J. & Headington, J. T. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br. J. Dermatol. 121, 421–431 (1989).
Nestle, F. O., Turka, L. A. & Nickoloff, B. J. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of TH1 type cytokines. J. Clin. Invest. 94, 202–209 (1994).
Wollenberg, A. et al. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Invest. Dermatol. 119, 1096–1102 (2002).
Kauffman, C. L. et al. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J. Invest. Dermatol. 123, 1037–1044 (2004).
Sano, S. et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nature Med. 11, 43–49 (2005).
Dauer, D. J. et al. Stat3 regulates genes common to both wound healing and cancer. Oncogene 24, 3397–3408 (2005).
Brandrup, F., Hauge, M., Henningsen, K. & Eriksen, B. Psoriasis in an unselected series of twins. Arch. Dermatol. 114, 874–878 (1978).
Duffy, D. L., Spelman, L. S. & Martin, N. G. Psoriasis in Australian twins. J. Am. Acad. Dermatol. 29, 428–434 (1993).
Farber, E. M., Nall, M. L. & Watson, W. Natural history of psoriasis in 61 twin pairs. Arch. Dermatol. 109, 207–211 (1974).
Pisani, M. & Ruocco, V. 'Twin' psoriasis in monozygotic twins. Arch. Dermatol. 120, 1418–1419 (1984).
Brandrup, F., Holm, N., Grunnet, N., Henningsen, K. & Hansen, H. E. Psoriasis in monozygotic twins: variations in expression in individuals with identical genetic constitution. Acta Derm. Venereol. 62, 229–236 (1982).
Bowcock, A. M. Psoriasis genetics: the way forward. J. Invest. Dermatol. 122, xv–xvii (2004).
Gaffney, P. M. et al. A genome-wide search for susceptibility genes in human systemic lupus erythematosus sib-pair families. Proc. Natl Acad. Sci. USA 95, 14875–14879 (1998).
Davies, J. L. et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature 371, 130–136 (1994).
Jawaheer, D. et al. Screening the genome for rheumatoid arthritis susceptibility genes: a replication study and combined analysis of 512 multicase families. Arthritis Rheum. 48, 906–916 (2003).
van Heel, D. A. et al. Inflammatory bowel disease susceptibility loci defined by genome scan meta-analysis of 1952 affected relative pairs. Hum. Mol. Genet. 13, 763–770 (2004).
Tiilikainen, A., Lassus, A., Karvonen, J., Vartiainen, P. & Julin, M. Psoriasis and HLA-Cw6. Br. J. Dermatol. 102, 179–184 (1980). This was the first study that showed the association of psoriasis with HLA-Cw6 , the most highly associated classical HLA allele.
Jenisch, S. et al. Linkage disequilibrium analysis of familial psoriasis: identification of multiple disease-associated MHC haplotypes. Tissue Antigens 53, 135–146 (1999).
Nair, R. P. et al. Localization of psoriasis-susceptibility locus PSORS1 to a 60-kb interval telomeric to HLA-C. Am. J. Hum. Genet. 66, 1833–1844 (2000). This was the first description of a dense set of microsatellite markers covering the MHC class I region and localizing PSORS1 to a particular interval in the MHC class I region.
Orru, S., Giuressi, E., Carcassi, C., Casula, M. & Contu, L. Mapping of the major psoriasis-susceptibility locus (PSORS1) in a 70-kb interval around the corneodesmosin gene (CDSN). Am. J. Hum. Genet. 76 164–171 (2004).
Veal, C. D. et al. Family-based analysis using a dense single-nucleotide polymorphism-based map defines genetic variation at PSORS1, the major psoriasis-susceptibility locus. Am. J. Hum. Genet. 71, 554–564 (2002).
Helms, C. et al. HLA class I associations and psoriasis susceptibility; an integration of classical markers and SNPs. Hum. Genet. (in the press).
Asumalahti, K. et al. Coding haplotype analysis supports HCR as the putative susceptibility gene for psoriasis at the MHC PSORS1 locus. Hum. Mol. Genet. 11, 589–597 (2002).
Asumalahti, K. et al. A candidate gene for psoriasis near HLA-C, HCR (Pg8), is highly polymorphic with a disease-associated susceptibility allele. Hum. Mol. Genet. 9, 1533–1542 (2000).
Elomaa, O. et al. Transgenic mouse models support HCR as an effector gene in the PSORS1 locus. Hum. Mol. Genet. 13, 1551–1561 (2004).
Allen, M. H. et al. A non-HLA gene within the MHC in psoriasis. Lancet 353, 1589–1590 (1999). This paper provided the first indication that a gene in the MHC class I region other than HLA-C is responsible for susceptibility to psoriasis.
Allen, M. et al. Corneodesmosin expression in psoriasis vulgaris differs from normal skin and other inflammatory skin disorders. Lab. Invest. 81, 969–976 (2001).
Tazi Ahnini, R. et al. Novel genetic association between the corneodesmosin (MHC S) gene and susceptibility to psoriasis. Hum. Mol. Genet. 8, 1135–1140 (1999).
Simon, M. et al. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J. Biol. Chem. 276, 20292–20299 (2001).
Jonca, N. et al. Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J. Biol. Chem. 277, 5024–5029 (2002).
Guerrin, M. et al. Identification of six novel polymorphisms in the human corneodesmosin gene. Tissue Antigens 57, 32–38 (2001).
Gallinaro, H. et al. A 4.2 kb upstream region of the human corneodesmosin gene directs site-specific expression in hair follicles and hyperkeratotic epidermis of transgenic mice. J. Invest. Dermatol. 122, 730–738 (2004).
Tomfohrde, J. et al. Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science 264, 1141–1145 (1994). This was the first study that indicated that a locus other than the MHC locus could contribute to susceptibility to psoriasis.
Helms, C. et al. A putative RUNX1 binding site variant between SLC9A3R1 and NAT9 is associated with susceptibility to psoriasis. Nature Genet. 35, 349–356 (2003). This study used family-based association mapping to identify a common variant for susceptibility to psoriasis that alters a RUNX-binding site and potentially affects immunological-synapse formation by altered regulation of SLC9A3R1.
Reczek, D., Berryman, M. & Bretscher, A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin–radixin–moesin family. J. Cell Biol. 139, 169–179 (1997).
Kurashima, K. et al. The apical Na+/H+ exchanger isoform NHE3 is regulated by the actin cytoskeleton. J. Biol. Chem. 274, 29843–29849 (1999).
Itoh, K. et al. Negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp–EBP50–ERM assembly. J. Immunol. 168, 541–544 (2002).
Bromley, S. K. et al. The immunological synapse. Annu. Rev. Immunol. 19, 375–396 (2001).
Davis, S. J. et al. The nature of molecular recognition by T cells. Nature Immunol. 4, 217–224 (2003).
Davidson, D., Bakinowski, M., Thomas, M. L., Horejsi, V. & Veillette, A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol. Cell. Biol. 23, 2017–2028 (2003).
Kim, D. H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002).
Hara, K. et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 (2002).
Capon, F. et al. Genetic analysis of PSORS2 markers in a UK dataset supports the association between RAPTOR SNPs and familial psoriasis. J. Med. Genet. 41, 459–460 (2004).
Hwu, W. L. et al. Mapping of psoriasis to 17q terminus. J. Med. Genet. 42, 152–158 (2005).
Capon, F. et al. Searching for psoriasis susceptibility genes in Italy: genome scan and evidence for a new locus on chromosome 1. J. Invest. Dermatol. 112, 32–35 (1999).
Bhalerao, J. & Bowcock, A. M. The genetics of psoriasis: a complex disorder of the skin and immune system. Hum. Mol. Genet. 7, 1537–1545 (1998).
Eckert, R. L. et al. S100 proteins in the epidermis. J. Invest. Dermatol. 123, 23–33 (2004).
De Heller-Milev, M., Huber, M., Panizzon, R. & Hohl, D. Expression of small proline rich proteins in neoplastic and inflammatory skin diseases. Br. J. Dermatol. 143, 733–740 (2000).
Marshall, D., Hardman, M. J., Nield, K. M. & Byrne, C. Differentially expressed late constituents of the epidermal cornified envelope. Proc. Natl Acad. Sci. USA 98, 13031–13036 (2001).
Hewett, D. et al. Identification of a psoriasis susceptibility candidate gene by linkage disequilibrium mapping with a localized single nucleotide polymorphism map. Genomics 79, 305–314 (2002). This was the first example of potential identification of a psoriasis-susceptibility gene by association studies with SNPs.
Hebert, S. C., Mount, D. B. & Gamba, G. Molecular physiology of cation-coupled Cl- cotransport: the SLC12 family. Pflugers Arch. 447, 580–593 (2004).
Tokuhiro, S. et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nature Genet. 35, 341–348 (2003).
Peltekova, V. D. et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nature Genet. 36, 471–475 (2004).
Gisler, S. M. et al. PDZK1: I. A major scaffolder in brush borders of proximal tubular cells. Kidney Int. 64, 1733–1745 (2003).
Becker, K. G. et al. Clustering of non-major histocompatibility complex susceptibility candidate loci in human autoimmune diseases. Proc. Natl Acad. Sci. USA 95, 9979–9984 (1998). This study indicates that there is an overlap in the locations of disease-susceptibility loci for several autoimmune diseases, including psoriasis.
Lee, Y. A. et al. Genomewide scan in German families reveals evidence for a novel psoriasis-susceptibility locus on chromosome 19p13. Am. J. Hum. Genet. 67, 1020–1024 (2000).
Low, J. H. et al. Inflammatory bowel disease is linked to 19p13 and associated with ICAM-1. Inflamm. Bowel Dis. 10, 173–181 (2004).
Thompson, S. D. et al. A genome-wide scan for juvenile rheumatoid arthritis in affected sibpair families provides evidence of linkage. Arthritis Rheum. 50, 2920–2930 (2004).
Van Belzen, M. J. et al. A major non-HLA locus in celiac disease maps to chromosome 19. Gastroenterology 125, 1032–1041 (2003).
Vyshkina, T., Banisor, I., Shugart, Y. Y., Leist, T. P. & Kalman, B. Genetic variants of Complex I in multiple sclerosis. J. Neurol. Sci. 228, 55–64 (2005).
Nejentsev, S. et al. Association of intercellular adhesion molecule-1 gene with type 1 diabetes. Lancet 362, 1723–1724 (2003).
Low, J. H. et al. Inflammatory bowel disease is linked to 19p13 and associated with ICAM-1. Inflamm. Bowel Dis. 10, 173–181 (2004).
Sigurdsson, S. et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am. J. Hum. Genet. 76, 528–537 (2005).
Cookson, W. O. et al. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nature Genet. 27, 372–373 (2001). This was the first report that showed that there is an overlap between the genetic loci that confer susceptibility to psoriasis and another inflammatory skin disease, atopic dermatitis.
Karason, A. et al. A susceptibility gene for psoriatic arthritis maps to chromosome 16q: evidence for imprinting. Am. J. Hum. Genet. 72, 125–131 (2003).
van Gisbergen, K. P., Paessens, L. C., Geijtenbeek, T. B. & van Kooyk, Y. Molecular mechanisms that set the stage for DC–T cell engagement. Immunol. Lett. 97, 199–208 (2005).
Millar, D. G. et al. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nature Med. 9, 1469–1476 (2003).
Begovich, A. B. et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 75, 330–337 (2004).
Bottini, N. et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nature Genet. 36, 337–338 (2004).
Kyogoku, C. et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am. J. Hum. Genet. 75, 504–507 (2004).
Latour, S. & Veillette, A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr. Opin. Immunol. 13, 299–306 (2001).
Wise, C. A. et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 11, 961–969 (2002).
Shoham, N. G. et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl Acad. Sci. USA 100, 13501–13506 (2003).
Prokunina, L. et al. Association of the PD-1.3A allele of the PDCD1 gene in patients with rheumatoid arthritis negative for rheumatoid factor and the shared epitope. Arthritis Rheum. 50, 1770–1773 (2004).
Ueda, H. et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423, 506–511 (2003).
Guo, D. et al. A functional variant of SUMO4, a new IκBα modifier, is associated with type 1 diabetes. Nature Genet. 36, 837–841 (2004).
Prokunina, L. et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nature Genet. 32, 666–669 (2002).
Lacaud, G. et al. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood 100, 458–466 (2002).
Brenner, O. et al. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc. Natl Acad. Sci. USA 101, 16016–16021 (2004).
Cook, P. W. et al. Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J. Clin. Invest. 100, 2286–2294 (1997).
Xia, Y. P. et al. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood 102, 161–168 (2003).
Kopp, T. et al. IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J. Immunol. 170, 5438–5444 (2003).
Kunstfeld, R. et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood 104, 1048–1057 (2004).
Carroll, J. M., Romero, M. R. & Watt, F. M. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 83, 957–968 (1995).
Hida, S. et al. CD8+ T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-α/β signaling. Immunity 13, 643–655 (2000).
Schon, M. P., Detmar, M. & Parker, C. M. Murine psoriasis-like disorder induced by naive CD4+ T cells. Nature Med. 3, 183–188 (1997).
Breban, M. et al. T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of HLA-B27 transgenic rats. J. Immunol. 156, 794–803 (1996).
Blumberg, H. et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell 104, 9–19 (2001).
Guo, L., Yu, Q. C. & Fuchs, E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 12, 973–986 (1993).
Acknowledgements
The authors acknowledge the invaluable contributions of the clinicians and patients who have contributed to this study. We thank M. Akin for tireless help in preparing this manuscript and A. Shaw for comments on figure 5. The studies carried out in our laboratories were supported, in part, by grants to A.M.B. and J.G.K. from the National Institutes of Health (United States) and to A.M.B. from the National Psoriasis Foundation (United States).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- PLAQUE
-
A raised, flat-topped lesion of the skin that is greater than or equal to 1 cm in diameter. This type of lesion typifies psoriasis vulgaris. Smaller lesions (known as papules) characterize guttate psoriasis. Pustular or erythrodermic forms of psoriasis might not show well-formed plaques but, instead, have pustules or extensive inflammation of the skin.
- CYCLOSPORIN
-
A commonly used immunosuppressive drug that blocks calcineurin A and thereby inhibits Tcell activation. It is used to prevent the rejection of transplanted organs and to treat some inflammatory diseases.
- SEVERE COMBINED IMMUNODEFICIENT MOUSE
-
(SCID mouse). An inbred mouse that has a naturally occurring mutation that impairs the development of B and T cells.
- AGR129 MOUSE
-
A mouse that is deficient in type-I- and type-II-interferon receptors and recombination-activating gene 2. These mice lack functional B and T cells and have impaired interferon-mediated innate immune functions. They have been used in transplantation experiments to improve graft acceptance.
- RETE
-
Regions of the interfollicular epidermis that project down into the dermis. These regions are also known as rete pegs. In normal skin, the projections are shallow, giving a wavy pattern to the epidermal–dermal junction. In psoriasis, rete are highly elongated and form finger-like downward projections into the dermis.
- DESMOSOME CONNECTION
-
An adhesive junction that connects epithelial cells in stratified squamous tissues. These junctions are composed of multiple protein subunits and are the points where keratin filaments are inserted into the plasma membrane.
- DC LYSOSOMAL-ASSOCIATED MEMBRANE PROTEIN
-
(DC-LAMP). A membrane protein that is associated with lysosomes in dendritic cells (DCs) and is a marker of activated and/or mature DCs.
- PSORIATIC ARTHRITIS
-
A form of inflammatory arthritis that occurs in patients with psoriasis.
- PLASMACYTOID DC
-
A dendritic cell (DC) that lacks myeloid markers such as CD11c and CD33 but expresses high levels of HLA-DR and CD123. These cells produce high levels of interferon-α after activation: for example, when stimulated through Toll-like receptors.
- CONCORDANCE
-
The occurrence of the same trait in both members of a pair of twins.
- LINKAGE DISEQUILIBRIUM
-
The non-random association of alleles from two independent loci, owing to their close physical proximity and a lack of recombination between them.
- DERMAL PAPILLA
-
The dermis is divided into a superficial region, known as the papillary dermis, and a deeper region, known as the reticular dermis. Portions of the papillary dermis that fill the space between the rete of the epidermis are known as dermal papillae. In normal skin, dermal papillae form shallow waves of upward projections. By contrast, in individuals with psoriasis, the papillae are long, finger-like upward projections.
- DESQUAMATION
-
The normal process through which terminally differentiated squamous keratinocytes (which are present in the cornified layer) are shed into the environment.
- ODDS RATIO
-
The ratio of the odds of an event occurring in each of two different groups. For example, the odds of individuals with psoriasis having a given allele compared with normal control individuals having the same allele.
- EPIDERMAL DIFFERENTIATION COMPLEX
-
(EDC). A group of ∼70 genes that is located on chromosome 1q21. These genes are expressed in terminal stages of keratinocyte differentiation. Some of the gene products generate differentiation-defining structures in granular and cornified keratinocytes.
- SHARED EPITOPE
-
A region in two or more unrelated molecules that has antigenic similarity.
- SINGLE-NUCLEOTIDE POLYMORPHISM
-
(SNP). A SNP indicates a particular site in the genome where more than one base can exist. In general, alleles of any polymorphism are present at a frequency of 1% or greater in the human genome.
Rights and permissions
About this article
Cite this article
Bowcock, A., Krueger, J. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol 5, 699–711 (2005). https://doi.org/10.1038/nri1689
Issue Date:
DOI: https://doi.org/10.1038/nri1689
This article is cited by
-
Identification immune response genes in psoriasis after treatment with secukinumab
BMC Medical Genomics (2023)
-
Network analysis of potential risk genes for psoriasis
Hereditas (2021)
-
First-in-class topical therapeutic omilancor ameliorates disease severity and inflammation through activation of LANCL2 pathway in psoriasis
Scientific Reports (2021)
-
Risk of Psoriasis Following Terbinafine or Itraconazole Treatment for Onychomycosis: A Population-Based Case-Control Comparative Study
Drug Safety (2018)