Key Points
-
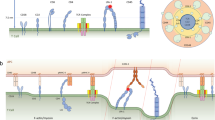
The onset and regulation of a specific immune response results from communication between T cells and antigen-presenting cells (APCs), which form a molecular cell–cell contact that is known as the immunological synapse.
-
Initially, the immunological synapse was viewed as a stereotypical adhesion and signalling device with a defined molecular structure and signalling processes.
-
However, as we discuss in this article, T cell–APC interactions comprise a diverse range of contact modes and distinct molecular arrangements.
-
The diversity of interaction modes might define a molecular code, which uses the different timing, spacing and molecular compositions of signalling platforms to determine the outcome of T cell–APC interactions.
Abstract
The onset and regulation of a specific immune response results from communication between T cells and antigen-presenting cells (APCs), which form molecular interactions at the site of cell–cell contact — and this is known as the immunological synapse. Initially, the immunological synapse was viewed as a stereotypical adhesion and signalling device with a defined molecular structure and signalling processes. However, as we discuss here, T-cell–APC interactions comprise a diverse range of contact modes and distinct molecular arrangements. These diverse interaction modes might define a molecular code, in which the differences in timing, spacing and molecular composition of the signalling platform determine the outcome of T-cell–APC interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
von Andrian, U. H. & Mackay, C. R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343, 1020–1034 (2000).
Friedl, P. & Storim, J. Diversity in immune cell interactions: states and functions of the immunological synapse. Trends Cell Biol. 14, 557–567 (2004).
Bromley, S. K. et al. The immunological synapse. Annu. Rev. Immunol. 19, 375–396 (2001).
Bossi, G. et al. The secretory synapse: the secrets of a serial killer. Immunol. Rev. 189, 152–160 (2002).
Norcross, M. A. A synaptic basis for T-lymphocyte activation. Ann. Immunol. (Paris) 135D, 113–134 (1984).
Geiger, B., Rosen, D. & Berke, G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J. Cell Biol. 95, 137–143 (1982).
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Wulfing, C. & Davis, M. M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 282, 2266–2269 (1998).
Negulescu, P. A., Krasieva, T. B., Khan, A., Kerschbaum, H. H. & Cahalan, M. D. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity 4, 421–430 (1996).
Valitutti, S., Dessing, M., Aktories, K., Gallati, H. & Lanzavecchia, A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J. Exp. Med. 181, 577–584 (1995).
Mempel, T. R., Henrickson, S. E. & Von Andrian, U. H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Miller, M. J., Hejazi, A. S., Wei, S. H., Cahalan, M. D. & Parker, I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl Acad. Sci. USA 101, 998–1003 (2004).
Miller, M. J., Safrina, O., Parker, I. & Cahalan, M. D. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 200, 847–856 (2004). References 11–13 provide compelling examples of different types and phases of T-cell activation initiated by DCs, as observed by intravital microscopy of lymph nodes. As well as generating visually stunning movies and three-dimensional reconstructions, these studies provide a precise map of interaction kinetics and duration in the course of productive and non-productive interactions between T cells and DCs.
Lee, K. H. et al. The immunological synapse balances T cell receptor signaling and degradation. Science 302, 1218–1222 (2003).
Kupfer, A., Swain, S. L., Janeway, C. A. Jr & Singer, S. J. The specific direct interaction of helper T cells and antigen-presenting B cells. Proc. Natl Acad. Sci. USA 83, 6080–6083 (1986).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Miller, M. J., Wei, S. H., Parker, I. & Cahalan, M. D. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 (2002).
Trautmann, A. & Valitutti, S. The diversity of immunological synapses. Curr. Opin. Immunol. 15, 249–254 (2003). This is a thought-provoking review and was the first to provide an overview of the diverse T-cell–APC interaction modes.
Hurez, V. et al. Restricted clonal expression of IL-2 by naive T cells reflects differential dynamic interactions with dendritic cells. J. Exp. Med. 198, 123–132 (2003).
Benvenuti, F. et al. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J. Immunol. 172, 292–301 (2004).
Al-Alwan, M. M., Rowden, G., Lee, T. D. & West, K. A. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J. Immunol. 166, 1452–1456 (2001).
Depoil, D. et al. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity 22, 185–194 (2005). T H 1 and T H 2 cells can engage B cells either simultaneously or sequentially, sensing and responding to different antigen loads, thereby forming simultaneous or serial immunological synapses. This report extends the view of the immunological synapse as a rapidly adapting communication device during T H -cell function.
Wetzel, S. A., McKeithan, T. W. & Parker, D. C. Live-cell dynamics and the role of costimulation in immunological synapse formation. J. Immunol. 169, 6092–6101 (2002).
Bousso, P., Bhakta, N. R., Lewis, R. S. & Robey, E. Dynamics of thymocyte–stromal cell interactions visualized by two-photon microscopy. Science 296, 1876–1880 (2002).
Bhakta, N. R., Oh, D. Y. & Lewis, R. S. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nature Immunol. 6, 143–151 (2005). During positive selection, thymocytes interact with peptide-loaded thymic stromal cells, receive a strong calcium signal and become temporarily immobilized. As well as prompting signal transduction, the calcium signal might cause migratory arrest and prolong thymocyte engagement with APCs.
Friedl, P. & Brocker, E. B. TCR triggering on the move: diversity of T-cell interactions with antigen-presenting cells. Immunol. Rev. 186, 83–89 (2002). This review describes the unifying concept of T-cell migration and signalling during interactions between T cells and APCs (that is, the dynamic immunological synapse).
Gunzer, M. et al. A spectrum of biophysical interaction modes between T cells and different antigen-presenting cells during priming in 3-D collagen and in vivo. Blood 104, 2801–2809 (2004). Using in vitro and intravital imaging of primary T-cell activation, direct comparison shows that the type of APC determines the interaction mode and its efficiency. Naive T cells establish short-lived contacts with DCs and activated B cells, whereas naive B cells are stably bound. Intriguingly, the efficiency of T-cell activation is inversely correlated with the duration of contact.
Iezzi, G., Karjalainen, K. & Lanzavecchia, A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8, 89–95 (1998).
Hugues, S. et al. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nature Immunol. 5, 1235–1242 (2004). For the generation of effector cells, a stable phase of T-cell–DC interaction is required. Conversely, under tolerizing conditions, the stable phase is absent, and only short-lived interactions occur; this initially leads to the proliferation of T cells, but it is followed by systemic tolerance.
Wulfing, C. et al. Kinetics and extent of T cell activation as measured with the calcium signal. J. Exp. Med. 185, 1815–1825 (1997).
Bousso, P. & Robey, E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nature Immunol. 4, 579–585 (2003).
Wulfing, C. et al. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nature Immunol. 3, 42–47 (2002).
Gunzer, M. et al. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity 13, 323–332 (2000).
Stoll, S., Delon, J., Brotz, T. M. & Germain, R. N. Dynamic imaging of T cell–dendritic cell interactions in lymph nodes. Science 296, 1873–1876 (2002).
Krummel, M. F., Sjaastad, M. D., Wulfing, C. & Davis, M. M. Differential clustering of CD4 and CD3ζ during T cell recognition. Science 289, 1349–1352 (2000).
Lindquist, R. L. et al. Visualizing dendritic cell networks in vivo. Nature Immunol. 5, 1243–1250 (2004). This study provides a fresh view on lymph-node anatomy, showing that there are relatively stable DC networks in the T-cell zone that function as scaffolding for the trafficking of T cells and the presentation of antigen.
Westermann, J. et al. Naive, effector, and memory T lymphocytes efficiently scan dendritic cells in vivo: contact frequency in T cell zones of secondary lymphoid organs does not depend on LFA-1 expression and facilitates survival of effector T cells. J. Immunol. 174, 2517–2524 (2005).
Jacobelli, J., Andres, P. G., Boisvert, J. & Krummel, M. F. New views of the immunological synapse: variations in assembly and function. Curr. Opin. Immunol. 16, 345–352 (2004).
Dustin, M. L., Bromley, S. K., Kan, Z., Peterson, D. A. & Unanue, E. R. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc. Natl Acad. Sci. USA 94, 3909–3913 (1997).
Zal, T., Zal, M. A. & Gascoigne, N. R. Inhibition of T cell receptor–coreceptor interactions by antagonist ligands visualized by live FRET imaging of the T-hybridoma immunological synapse. Immunity 16, 521–534 (2002).
Donnadieu, E. et al. Imaging early steps of human T cell activation by antigen-presenting cells. J. Immunol. 148, 2643–2653 (1992).
Freiberg, B. A. et al. Staging and resetting T cell activation in SMACs. Nature Immunol. 3, 911–917 (2002).
Huppa, J. B., Gleimer, M., Sumen, C. & Davis, M. M. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nature Immunol. 4, 749–755 (2003).
Lee, K. H. et al. T cell receptor signaling precedes immunological synapse formation. Science 295, 1539–1542 (2002).
Bonello, G. et al. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J. Cell Sci. 117, 1009–1016 (2004).
Horejsi, V., Zhang, W. & Schraven, B. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nature Rev. Immunol. 4, 603–616 (2004).
Harriague, J. & Bismuth, G. Imaging antigen-induced PI3K activation in T cells. Nature Immunol. 3, 1090–1096 (2002).
Costello, P. S., Gallagher, M. & Cantrell, D. A. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nature Immunol. 3, 1082–1089 (2002).
Stradal, T. E. et al. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 14, 303–311 (2004).
Moss, W. C., Irvine, D. J., Davis, M. M. & Krummel, M. F. Quantifying signaling-induced reorientation of T cell receptors during immunological synapse formation. Proc. Natl Acad. Sci. USA 99, 15024–15029 (2002).
Holdorf, A. D., Lee, K. H., Burack, W. R., Allen, P. M. & Shaw, A. S. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nature Immunol. 3, 259–264 (2002).
Das, V. et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity 20, 577–588 (2004).
Villalba, M. et al. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J. Cell Biol. 155, 331–338 (2001).
Cherry, L. K., Li, X., Schwab, P., Lim, B. & Klickstein, L. B. RhoH is required to maintain the integrin LFA-1 in a nonadhesive state on lymphocytes. Nature Immunol. 5, 961–967 (2004).
Liu, L. et al. The GTPase Rap1 regulates phorbol 12-myristate 13-acetate-stimulated but not ligand-induced β1 integrin-dependent leukocyte adhesion. J. Biol. Chem. 277, 40893–40900 (2002).
Eibert, S. M. et al. Cofilin peptide homologs interfere with immunological synapse formation and T cell activation. Proc. Natl Acad. Sci. USA 101, 1957–1962 (2004).
Andres, P. G. et al. CD28 signals in the immature immunological synapse. J. Immunol. 172, 5880–5886 (2004).
Dustin, M. L. et al. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94, 667–677 (1998).
Egen, J. G. & Allison, J. P. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 16, 23–35 (2002).
Purtic, B., Pitcher, L. A., van Oers, N. S. & Wulfing, C. T cell receptor (TCR) clustering in the immunological synapse integrates TCR and costimulatory signaling in selected T cells. Proc. Natl Acad. Sci. USA 102, 2904–2909 (2005).
Maldonado, R. A., Irvine, D. J., Schreiber, R. & Glimcher, L. H. A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature 431, 527–532 (2004). This study shows the position and functional relevance of IFN-γR in the immunological synapse, indicating that IFN-γR has an immediate early role in polarization towards T H -cell phenotypes.
Jordan, S. & Rodgers, W. T cell glycolipid-enriched membrane domains are constitutively assembled as membrane patches that translocate to immune synapses. J. Immunol. 171, 78–87 (2003).
Poenie, M., Kuhn, J. & Combs, J. Real time visualization of the cytoskeleton and effector functions in T cells. Curr. Opin. Immunol. 16, 428–438 (2004).
Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Liu, H., Rhodes, M., Wiest, D. L. & Vignali, D. A. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 13, 665–675 (2000).
Mazerolles, F., Barbat, C., Hivroz, C. & Fischer, A. Phosphatidylinositol 3-kinase participates in p56lck/CD4-dependent down-regulation of LFA-1-mediated T cell adhesion. J. Immunol. 157, 4844–4854 (1996).
Ebert, L. M. & McColl, S. R. Up-regulation of CCR5 and CCR6 on distinct subpopulations of antigen-activated CD4+ T lymphocytes. J. Immunol. 168, 65–72 (2002).
Allenspach, E. J. et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15, 739–750 (2001).
Potter, T. A., Grebe, K., Freiberg, B. & Kupfer, A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc. Natl Acad. Sci. USA 98, 12624–12629 (2001).
Faroudi, M. et al. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. Proc. Natl Acad. Sci. USA 100, 14145–14150 (2003).
Purbhoo, M. A., Irvine, D. J., Huppa, J. B. & Davis, M. M. T cell killing does not require the formation of a stable mature immunological synapse. Nature Immunol. 5, 524–530 (2004).
O'Keefe, J. P., Blaine, K., Alegre, M. L. & Gajewski, T. F. Formation of a central supramolecular activation cluster is not required for activation of naive CD8+ T cells. Proc. Natl Acad. Sci. USA 101, 9351–9356 (2004).
Kuhn, J. R. & Poenie, M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity 16, 111–121 (2002).
Kupfer, A., Mosmann, T. R. & Kupfer, H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc. Natl Acad. Sci. USA 88, 775–779 (1991).
Reichert, P., Reinhardt, R. L., Ingulli, E. & Jenkins, M. K. In vivo identification of TCR redistribution and polarized IL-2 production by naive CD4 T cells. J. Immunol. 166, 4278–4281 (2001).
Richie, L. I. et al. Imaging synapse formation during thymocyte selection: inability of CD3ζ to form a stable central accumulation during negative selection. Immunity 16, 595–606 (2002).
Hailman, E., Burack, W. R., Shaw, A. S., Dustin, M. L. & Allen, P. M. Immature CD4+CD8+ thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity 16, 839–848 (2002).
Zaru, R., Cameron, T. O., Stern, L. J., Muller, S. & Valitutti, S. TCR engagement and triggering in the absence of large-scale molecular segregation at the T cell–APC contact site. J. Immunol. 168, 4287–4291 (2002).
Blanchard, N. et al. Strong and durable TCR clustering at the T/dendritic cell immune synapse is not required for NFAT activation and IFN-γ production in human CD4+ T cells. J. Immunol. 173, 3062–3072 (2004).
Revy, P., Sospedra, M., Barbour, B. & Trautmann, A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nature Immunol. 2, 925–931 (2001). This paper provides evidence that non-cognate T-cell–APC interactions elicit (weak) tyrosine phosphorylation at the plasma membrane and calcium influx, but they do not induce full activation of T cells. Together with reference 12, these findings implicate such signals in supporting the survival of T cells and in maintaining a peripheral pool of naive T cells (that is, in homeostasis).
Valitutti, S., Müller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature 375, 148–151 (1995).
Friedl, P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 16, 14–23 (2004).
Friedl, P., Entschladen, F., Conrad, C., Niggemann, B. & Zanker, K. S. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize β1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur. J. Immunol. 28, 2331–2343 (1998).
Sanchez-Madrid, F. & del Pozo, M. A. Leukocyte polarization in cell migration and immune interactions. EMBO J. 18, 501–511 (1999).
Kupfer, A. & Singer, S. J. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu. Rev. Immunol. 7, 309–337 (1989).
Ingulli, E., Mondino, A., Khoruts, A. & Jenkins, M. K. In vivo detetction of dendritic cell antigen presentation to CD4+ T cells. J. Exp. Med. 185, 2133–2141 (1997).
Friedl, P. & Gunzer, M. Interaction of T cells with APCs: the serial encounter model. Trends Immunol. 22, 187–191 (2001).
Faroudi, M., Zaru, R., Paulet, P., Muller, S. & Valitutti, S. T lymphocyte activation by repeated immunological synapse formation and intermittent signaling. J. Immunol. 171, 1128–1132 (2003).
Liu, K. et al. Augmentation in expression of activation-induced genes differentiates memory from naive CD4+ T cells and is a molecular mechanism for enhanced cellular response of memory CD4+ T cells. J. Immunol. 166, 7335–7344 (2001).
Yasutomo, K., Doyle, C., Miele, L., Fuchs, C. & Germain, R. N. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature 404, 506–510 (2000).
Liu, X. & Bosselut, R. Duration of TCR signaling controls CD4–CD8 lineage differentiation in vivo. Nature Immunol. 5, 280–288 (2004).
Brocker, T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class-II expressing dendritic cells. J. Exp. Med. 186, 1223–1232 (1997).
Li, Q. J. et al. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nature Immunol. 5, 791–799 (2004).
Boes, M. et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418, 983–988 (2002).
Okada, T. M. et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 3, e150 (2005).
Byersdorfer, C. A., Dipaolo, R. J., Petzold, S. J. & Unanue, E. R. Following immunization antigen becomes concentrated in a limited number of APCs including B cells. J. Immunol. 173, 6627–6634 (2004).
Kedl, R. M. et al. T cells compete for access to antigen-bearing antigen-presenting cells. J. Exp. Med. 192, 1105–1113 (2000).
Zinkernagel, R. M. et al. T and B cell tolerance and responses to viral antigens in transgenic mice: implications for the pathogenesis of autoimmune versus immunopathological disease. Immunol. Rev. 122, 133–171 (1991).
Radoja, S. et al. CD8+ tumor-infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J. Immunol. 167, 5042–5051 (2001).
Hart, D. N. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90, 3245–3287 (1997).
Cella, M., Engering, A., Pinet, V., Pieters, J. & Lanzavecchia, A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388, 782–787 (1997).
Krummel, M. F. & Davis, M. M. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr. Opin. Immunol. 14, 66–74 (2002).
Al-Alwan, M. M. et al. Dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J. Immunol. 171, 4479–4483 (2003).
Schweitzer, B. et al. Multiplexed protein profiling on microarrays by rolling-circle amplification. Nature Biotechnol. 20, 359–365 (2002).
Wang, J. P., Rought, S. E., Corbeil, J. & Guiney, D. G. Gene expression profiling detects patterns of human macrophage responses following Mycobacterium tuberculosis infection. FEMS Immunol. Med. Microbiol. 39, 163–172 (2003).
Klein, U. et al. Transcriptional analysis of the B cell germinal center reaction. Proc. Natl Acad. Sci. USA 100, 2639–2644 (2003).
Springer, T. A. Adhesion receptors of the immune system. Nature 346, 425–434 (1990).
Ford, G. S., Barnhart, B., Shone, S. & Covey, L. R. Regulation of CD154 (CD40 ligand) mRNA stability during T cell activation. J. Immunol. 162, 4037–4044 (1999).
Underhill, D. M., Bassetti, M., Rudensky, A. & Aderem, A. Dynamic interactions of macrophages with T cells during antigen presentation. J. Exp. Med. 190, 1909–1914 (1999).
Mempel, T. R., Scimone, M. L., Mora, J. R. & von Andrian, U. H. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr. Opin. Immunol. 16, 406–417 (2004).
Wei, S. H., Parker, I., Miller, M. J. & Cahalan, M. D. A stochastic view of lymphocyte motility and trafficking within the lymph node. Immunol. Rev. 195, 136–159 (2003).
Irvine, D. J., Purbhoo, M. A., Krogsgaard, M. & Davis, M. M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Filipp, D. et al. Regulation of Fyn through translocation of activated Lck into lipid rafts. J. Exp. Med. 197, 1221–1227 (2003).
Krogsgaard, M. & Davis, M. M. How T cells 'see' antigen. Nature Immunol. 6, 239–245 (2005).
Krause, M. et al. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 149, 181–194 (2000).
Vicente-Manzanares, M. & Sanchez-Madrid, F. Role of the cytoskeleton during leukocyte responses. Nature Rev. Immunol. 4, 110–122 (2004).
Serrador, J. M. et al. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity 20, 417–428 (2004).
Acknowledgements
We acknowledge M. Jobberger for carrying out scanning electron microscopy and T. Stradal, B. Schraven and M. Davis for helpful comments and discussion. This work was supported by grants from the Deutsche Forschungsgemeinschaf (Germany) to P.F. and M.G., and from the Dutch Cancer Society to A.Th.d.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- UROPOD
-
The posterior tail of migrating amoeboid cells. It is rich in filamentous actin, microtubules, and cytoskeletal adaptor proteins (such as ezrin and moesin), as well as adhesion molecules (such as CD43 and CD44) and lipid rafts.
- LEADING LAMELLIPODIUM
-
An actin-rich membrane protrusion that extends, retracts and generates physical traction towards the underlying substrate in the process of cell migration.
- BLAST
-
An immune cell in a proliferating state, as shown by an enlarged cytoplasm and nucleus (assessed by blood smears or flow cytometry). A T lymphoblast has entered the S (synthesis) and G2 (gap 2) phases of the cell cycle, and under activating conditions, it develops into an effector T cell or a memory T cell. Under tolerizing conditions, a T lymphoblast can either become anergic or undergo apoptosis through activation-induced cell death.
- AGONIST PEPTIDE
-
A peptide that mimics cognate antigen and results in T-cell activation and proliferation. Agonistic activity is often, but not always, associated with high-affinity and/or avidity binding of TCR to peptide–MHC complexes.
- ANTAGONIST PEPTIDE
-
A peptide that prevents T-cell activation by cognate antigen, either by competition for TCR sites or by active delivery of negative signals.
- PERIPHERAL INTERSTITIAL TISSUES
-
Immune cells constitutively enter and migrate through all parenchymatous tissues and organs (which are composed of a loose fibrillar extracellular matrix and the parenchymal cells contained within it), including the skin, kidneys, thyroid gland, liver and lungs. Under non-inflammatory conditions, regions that are excluded from T-cell trafficking are the bone, brain, vitreous body of the eye and parts of the testes.
- FLUORESCENCE RESONANCE ENERGY TRANSFER
-
(FRET). A technique that is used to measure protein–protein interactions either by microscopy or flow cytometry. Proteins fused to cyan, yellow or red fluorescent proteins are expressed and assessed for interaction by measuring the energy transfer between fluorophores, which can only occur if proteins physically interact. FRET can also be used to examine the activation state of certain proteins if their activation results in a change in the conformation of the protein.
- IMMUNORECEPTOR TYROSINE-BASED ACTIVATION MOTIF
-
(ITAM). A sequence that is present in the cytoplasmic domains of the invariant chains that are associated with various cell-surface immune receptors, such as the T-cell receptor, the B-cell receptor, the receptor for IgE (FcεR) and natural-killer-cell activating receptors, as well as in some signalling molecules that are immediately downstream. Following phosphorylation of the tyrosine residue, ITAMs function as docking sites for SRC homology 2 (SH2)-domain-containing tyrosine kinases and adaptor molecules, thereby facilitating intracellular-signalling cascades.
- SRC-HOMOLOGY-2 DOMAIN
-
(SH2 domain). A protein domain that is commonly found in signal-transduction molecules. It specifically recognizes phosphotyrosine-containing peptide sequences in proteins.
- PLECKSTRIN-HOMOLOGY DOMAIN
-
A protein–lipid interaction domain that usually consists of 100 amino-acid residues. These domains have little overall sequence homology but have conserved motifs and tertiary structure. They are thought to be involved in the anchoring of proteins to the membrane and have been found to bind the following: phospholipids (including phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate), proteins (including the β- and γ-subunit of heterotrimeric G proteins), and phosphorylated serine or threonine residues.
- LIPID RAFT
-
An area of the plasma membrane that is rich in cholesterol, glycosphingolipids, several signalling proteins — such as SRC-family kinases, RAS, LAT (linker for activation of T cells) and PAG (protein associated with glycolipid-enriched microdomains) — and glycosylphosphatidylinositol-anchored proteins. These domains are also known as glycolipid-enriched microdomains (GEMs) and detergent-insoluble glycolipid-enriched membranes (DIGs).
- SUPRAMOLECULAR ACTIVATION CLUSTER
-
(SMAC). A membrane region that is enriched in (clustered) TCR, adhesion molecules and/or signalling molecules, as detected by fluorescence microscopy. SMACs are thought to be focalized regions of receptor–ligand interaction and signal transduction. Their known size ranges from ∼150 nm to several millimetres in diameter. Because of the limited resolution of light microscopy, neighbouring aggregates of less than ∼150 nm cannot be discriminated as individual objects and therefore appear as 'diffusely' distributed. The number, size and function of very small clusters therefore remains unknown.
- GRANZYMES
-
Secreted serine proteases that enter target cells by a receptor-mediated endocytic pathway, then cleave and activate intracellular caspases, leading to target-cell apoptosis.
- PERFORIN
-
A secreted protein that supports the cytotoxic function of granzymes in the target cell. After being internalized by the target cell, perforin disrupts the endosomal membrane and mediates the transport of granzymes into the cytoplasm.
- NEGATIVE SELECTION
-
A step in the process of T-cell differentiation in the thymus. T cells that express T-cell receptors with high affinity for self-antigens are eliminated from the repertoire by apoptosis after recognition of their target antigen presented by thymic medullary epithelial or dendritic cells.
- OPTICAL REPORTER ASSAY
-
T cells that express a fluorescent protein (such as green fluorescent protein) under the control of a promoter of a gene involved in T-cell-receptor activation fluoresce if activation occurs. Useful reporter constructs for determining activation are the interleukin-2 (IL-2) promoter, for initial activation, and the IL-4 or interferon-γ promoter, for T-cell effector function.
- POSITIVE SELECTION
-
A step in the process of T-cell differentiation in the thymus. T cells that express T-cell receptors with moderate to high affinity for self-antigens receive a survival signal and continue to develop towards becoming double positive (CD4+CD8+) T cells. Positive selection occurs through antigens presented by resident stromal cells and dendritic cells in the thymic cortex and is followed by negative selection.
- HOMEOSTASIS
-
The maintenance of relatively stable numbers of peripheral T cells. Naive CD4+ and CD8+ T cells recirculate between the blood and secondary lymphoid organs, where they receive survival signals.
- TH1-CELL PHENOTYPE
-
(T–helper-1 cell phenotype). A T helper cell that mainly secretes interleukin-2 and interferon-γ (IFN-γ). These cells therefore support cell-mediated immune responses, such as activation of cytotoxic T cells, IFN-mediated killing of virus-infected cells and activation of the monocyte/macrophage system.
- TWO-PHOTON INTRAVITAL MICROSCOPY
-
Laser-scanning microscopy that uses pulsed infrared laser light for the excitation of conventional fluorophores or fluorescent proteins. The main advantage is deep tissue penetration of the infrared light, owing to the low level of light scattering within the tissue.
Rights and permissions
About this article
Cite this article
Friedl, P., den Boer, A. & Gunzer, M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat Rev Immunol 5, 532–545 (2005). https://doi.org/10.1038/nri1647
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1647
This article is cited by
-
Current understanding of biological identity at the nanoscale and future prospects
Nature Nanotechnology (2021)
-
A carboxymethyl dextran-based polymeric conjugate as the antigen carrier for cancer immunotherapy
Biomaterials Research (2018)
-
A correlative and quantitative imaging approach enabling characterization of primary cell-cell communication: Case of human CD4+ T cell-macrophage immunological synapses
Scientific Reports (2018)
-
The infectious synapse formed between mature dendritic cells and CD4+T cells is independent of the presence of the HIV-1 envelope glycoprotein
Retrovirology (2013)
-
The adapter protein ADAP is required for selected dendritic cell functions
Cell Communication and Signaling (2012)