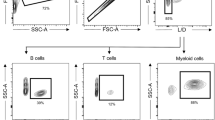
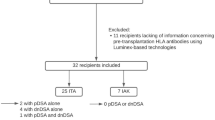
Key Points
-
Clinical islet allotransplantation has progressed slowly since improved islet-isolation technologies resulted in the achievement of insulin independence in the first series of successful islet allografts in 1990.
-
Insulin-independence rates have markedly improved as new and more effective combinations of immunosuppressive drugs have been introduced.
-
Because of the risks associated with life-long recipient immunosuppression, islet transplants are now limited to the most severe cases of type 1 diabetes.
-
For islet transplantation to become widely applicable, successful strategies for tolerance induction need to be developed.
Abstract
Type 1 diabetes mellitus results from autoimmune destruction of the insulin-secreting cells in the pancreas. Daily treatment with exogenous insulin is required, but because of difficulties in achieving physiological control of blood-glucose concentrations, chronic and degenerative complications still occur in a marked fraction of patients. Islet transplantation can normalize metabolic control in a way that has been virtually impossible to achieve with exogenous insulin, but life-long immunosuppression of the recipients is required, limiting the procedure to the most severe forms of diabetes. This article outlines the history of and recent progress in the field, as well as the present immunological challenges and possible strategies for tolerance induction that are crucial to make clinical islet transplantation more widely available.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Williams, P. W. Notes on diabetes treated with extract and by grafts of sheep's pancreas. Br. Med. J. 2, 1303–1304 (1894).
Minkowsky, O. Untersuchungen uber den diabetes mellitus nach extirpation des pankreas. Berl. Klin. Wschr. 29, 90 (1892).
Bliss, M. The Discovery of Insulin. The University of Chicago Press (1982).
The DCCT Research Group: the effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
Lacy, P. E. & Kostianovsk, M. A method for isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16, 35–39 (1967).
Ballinger, W. F. & Lacy, P. E. Transplantation of intact pancreatic islets in rats. Surgery 72, 175–186 (1972).
Hering, B. & Ricordi, C. Islet transplantation in type 1 diabetes: results, research priorities and reasons for optimism. Graft 2, 12–27 (1999).
Ricordi, C. The Automated Method for Islet Isolation, 99–112 (R. G. Landes Company Medical Publisher, CRC Press (Distr.), Austin, Berlin, 1992).
Ricordi, C., Lacy, P. E., Finke, E. H., Olack, B. J. & Scharp, D. W. Automated method for isolation of human pancreatic islets. Diabetes 37, 413–420 (1988). This was the original paper discussing the islet-isolation technology that was used in the first successful trials of islet transplantation.
Scharp, D. W. et al. Insulin independence after islet transplantation into type 1 diabetic patient. Diabetes 39, 515–518 (1990).
Tzakis, A. G. et al. Pancreatic islet transplantation after upper abdominal exenteration and liver replacement. Lancet 336, 402–405 (1990).
Ricordi, C. et al. Human islet isolation and allotransplantation in 22 consecutive cases. Transplantation 53, 407–414 (1992).
Ricordi, C. et al. Detection of pancreatic islet tissue following islet allotransplantation in man. Transplantation 52, 1079–1080 (1991).
Sever, C. E. et al. Islet cell allotransplantation in diabetic patients — histologic findings in four adults simultaneously receiving kidney or liver transplants. Am. J. Pathol. 140, 1255–1260 (1992).
Ricordi, C. Islet transplantation: a brave new world. Diabetes 52, 1595–1603 (2003). The text of the 2002 Outstanding Achievement Award Lilly Lecture delivered at the annual American Diabetes Association Congress, outlining major steps in islet isolation and transplantation, as well as current and future challenges.
Alejandro, R. et al. Long term function (6 years) of islet allografts in type 1 diabetes mellitus. Diabetes 46, 1983–1090 (1997).
Linetsky, E. et al. Improved human islet isolation using a new enzyme blend, Liberase™. Diabetes 46, 1120–1123 (1997).
Bertuzzi, F. et al. Successful transplantation of human islets in recipients bearing a kidney graft. Diabetologia 45, 77–84 (2002).
Shapiro, A. M. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 289–290 (2000). This report indicated that insulin independence could be achieved after islet transplantation using rapamycin-based immunosuppression.
Rodriguez & Rilo, H. Acceleration of chronic failure of intrahepatic canine islet autografts by a short course of prednisone. Transplantation 57, 181–187 (1994).
Ricordi, C. et al. In vivo effect of FK 506 on human pancreatic islets. Transplantation 52, 519–522 (1991).
Fernandez, L. A. et al. The effects of maintenance doses of FK506 versus cyclosporin A on glucose and lipid metabolism after orthotopic liver transplantation. Transplantation 68, 1532–1541 (1999).
Kenyon, N. S. et al. Long term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc. Natl Acad. Sci. USA 96, 8132–8137 (1999).
Kenyon, N. S. et al. Long-term survival and function of intrahepatic islet allografts in baboons treated with humanized anti-CD154. Diabetes 48, 1473–1481 (1999).
Ryan, E. A. et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes 51, 2148–2157 (2002).
Goss, J. A. et al. Achievement of insulin independence in three consecutive type 1 diabetic patients via pancreatic islet transplantation using islets isolated at a remote islet isolation center. Transplantation 74, 1761–1766 (2002).
Suchin, E. J. et al. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J. Immunol. 166, 973–981 (2001).
Sykes, M. Mixed chimerism and transplant tolerance. Immunity 14, 417–424 (2001).
Sayegh, M. H. et al. Immunologic tolerance to renal allografts after bone marrow transplantations from the same donors. Ann. Intern. Med. 114, 954–955 (1991).
Inverardi, L. et al. Targeted bone marrow radioablation with 153Samarium-Lexidronam promotes allogeneic hematopoietic chimerism and donor-specific immunological hyporesponsiveness. Transplantation (in the press).
O'Connell, P. J. et al. Unmodificed pancreatic islet allograft rejection results in the preferential expression of certain T cell transcripts. J. Immunol. 150, 1093–2104 (1993).
Sayegh, M. H. & Turka, L. A. The role of co-stimulatory activation pathways in transplant tolerance. N. Engl. J. Med. 338, 1813–1821 (1998).
Li, X. C., Zand, M. S., Li, Y., Zheng, X. X. & Strom, T. B. On histocompatibility barriers, TH1 to TH2 immune deviation, and the nature of the allgoraft responses. J. Immunol. 161, 2241–2247 (1998).
Sho, M. et al. Physiological mechanisms of regulating alloimmunity: cytokines, CTLA-4, CD25+ cells, and the alloreactive T cell clone size. J. Immunol. 169, 3744–3751 (2002).
Kishimoto, K. et al. TH1 cytokines, programmed cell death, and alloreactive T cell clone size in transplant tolerance. J. Clin. Invest. 109, 1471–1479 (2002). References 33–35 show that T helper 1 (T H 1) to T H 2 immune deviation or apoptotic depletion of alloreactive T cells alone can create tolerance to allogeneic grafts across minor, but not major, histocompatibility barriers.
Smith, J. A., Tso, J. Y., Clark, M. R., Cole, M. S. & Bluestone, J. A. Non-mitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J. Exp. Med. 185, 1413–1422 (1997).
Smith, J. A. & Bluestone, J. A. T cell inactivation and cytokine deviation and cytokine deviation promoted by anti-CD3 mAbs. Curr. Opin. Immunol. 9, 648–654 (1997).
Waldmann, H. Reprogramming the immune system. Immunol. Rev. 185, 227–235 (2002). This review emphasizes the importance of regulatory T cells in obtaining peripheral allograft tolerance.
Pullar, C. E., Morris, P. J. & Wood, K. J. Altered proximal T cell signaling events in mouse CD4+ T cells in the presence of anti-CD4 monoclonal antibodies: evidence for reduced phosphorylation of Zap-70 and LAT. Scand. J. Immunol. 57, 333–341 (2003).
Zheng, X. X. & Strom, T. B. IL-2 receptor targeted cytolytic IL-2/Fc fusion protein treatment blocks diabetogenic autoimmunity in non-obese diabetic mice. J. Immunol. 163, 4041–4048 (1999).
Mordes, J. P. & Rossini, A. A. Survival of mouse pancreatic islet transplant in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl Acad. Sci. USA 92, 9560–9564 (1995).
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell co-stimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Larsen, C. P. et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 381, 434–438 (1996).
Wells, A. D. et al. Requirement for T cell apoptosis in the induction of peripheral transplantation tolerance. Nature Med. 5, 1303–1307 (1999).
Dai, Z., Konieczny, B. T., Baddoura, F. K. & Lakkis, F. G. Impaired alloantigen-mediated T cell apoptosis and failure to induce long-term allograft survival in IL-2-deficient mice. J. Immunol. 161, 1659–1663 (1998).
Li, Y. et al. Blocking both signal 1 and signal 2 of T cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature Med. 5, 1298–1302 (1999). References 44–46 demonstrate the importance of preserving the apoptotic response of alloreactive T cells to create tolerance to MHC-mismatched allografts.
Wood, K. J. & Sakaguchi, S. Regulatory T cells in transplantation tolerance. Nature Rev. Immunol. 3, 199–210 (2003).
Sanchez-Fueyo, A., Weber, M., Domenig, C., Strom, T. B. & Zheng, X. X. Tracking the immunoregulatory mechanisms active during allograft response. J. Immunol. 168, 2274–2281 (2002).
Li, X. C., Wells, A. D., Strom, T. B. & Turka, L. A. T cell death and transplantation tolerance. Immunity 14, 407–416 (2001).
Zheng, X. X. et al. Favorably tipping the balance between cytopathic and regulatory T cells to create transplant tolerance. Immunity 19, 503–514 (2003).
Plain, K. M., Chen, J., Merten, S., He, X. Y. & Ball, B. M. Induction of specific tolerance to allografts in rats by therapy with non-mitogenic, non-depleting anti-CD3 monoclonal antibody: association with TH2 cytokines not anergy. Transplantation 67, 605–613 (1999).
Chatenoud, L., Thervet, E., Primo, J. & Bach, J. -F. Anti-CD3 antibody induces long-term remission of overt autoimmunity in non-obese diabetic mice. Proc. Natl Acad. Sci. USA 91, 123–127 (1994).
Chatenoud, L., Primo, J. & Bach, J. -F. CD3 antibody-induced dominant self tolerance in overtly autoimmunity in non-obese diabetic mice. J. Immunol. 158, 2947–2954 (1997).
Chatenoud, L. CD3-specific antibody-induced active tolerance. Nature Rev. Immunol. 3, 123–132 (2003).
Belghith, M. et al. TGF-β dependent mechanisms mediate restoration of self tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nature Med. 9, 1202–1208 (2003).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002).
Waldmann, T. A. & O'Shea, J. The use of antibodies against the IL-2 receptor in transplantation. Curr. Opin. Immunol. 10, 507–512 (1998).
Strom, T. B., Kelley, V. R., Murphy, J. R., Nichols, J. & Woodsworth, T. G. Interleukin-2 receptor-directed therapies: antibody- or cytokine-based targeting molecules. Annu. Rev. Immunol. 44, 343–353 (1993).
Marrack, P. et al. Homeostasis of αβTCR+ T cells. Nature Immunol. 1, 107–111 (2000).
Li, X. C. et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nature Med. 7, 114–118 (2001).
Ku, C. C., Murakami, M., Sakamoto, J., Kappler, J. & Marrack, P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 105–110 (2000).
Waldman, T. A., Dubois, S. & Tagaya, Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity 14, 105–110 (2001).
Lenardo, M. J. Interleukin-2 programs a mouse αβ T lympyhocytes for apoptosis. Nature 353, 858–861 (1991). References 60–63 identify opposing effects of interleukin-2 (IL-2) and IL-15 in delivering life or death signals to various T-cell subsets.
Papiernik, M., deMoraes, M. L., Pontoux, C., Vasseur, F. & Penit, C. Regulatory CD4 T cells: expression of IL-2Rα chain, resistance of clonal deletion and IL-2 dependency. Int. Immunol. 10, 371–378 (1998).
VanParijs, L. et al. Uncoupling IL-2 signals that regulate T cell proliferation, survival and Fas-mediated activation-induced cell death. Immunity 11, 281–288 (1998).
Malek, T. R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the non-redundant function of IL-2. Immunity 17, 167–178 (2002).
Graca, L. et al. Both CD4+CD25+ and CD4+CD25− regulatory cells mediate dominant transplantation tolerance. J. Immunol. 168, 5558–5565 (2002).
Kingsley, C. I., Karim, M., Bushell, A. R. & Wood, K. J. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4+ and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 168, 1080–1086 (2002).
Banz, A., Pontoux, C. & Papiernik, M. Modulation of Fas-dependent apoptosis: a dynamic process controlling both the persistence and death of CD4 regulatory T cells and effector T cells. J. Immunol. 169, 750–757 (2002).
Ferrari-Lacraz, S. et al. An antagonist IL-15/Fc protein prevents co-stimulation blockade-resistant rejection. J. Immunol. 167, 3478–3485 (2001).
Kim, Y. S. et al. Targeting the IL-15 receptor with an antagonist IL-15 mutant/Fcγ2a protein blocks delayed-type hypersensitivity. J. Immunol. 160, 5742–5748 (1999).
Pearson, T. et al. Genetic dissociation of autoimmunity and resistance to co-stimulation blockade-induced transplantation tolerance in non-obese diabetic mice. J. Immunol. 171, 185–195 (2003).
Harada, H. et al. The role of the ICOS–B7h T cell co-stimulatory pathway in transplantation immunity. J. Clin. Invest. 112, 234–243 (2003).
Yuan, X. et al. The role of the CD134–CD134 ligand co-stimulatory pathway in alloimmune responses in vivo. J. Immunol. 170, 2949–2955 (2003).
Demirci, G. et al. Critical role of OX40 in CD28 and CD154-independent rejection. J. Immunol. 172, 1691–1698 (2004).
Adams, A. B. et al. Calcineurin inhibitor-free CD28 blockade-based protocol protects allogeneic islets in nonhuman primates. Diabetes 51, 265–270 (2002).
Thomas, J. M. et al. Successful reversal of streptozotocin-induced diabetes with stable allogeneic islet function in a preclinical model of type 1 diabetes. Diabetes 50, 1227–1236 (2001).
Kawai, T. et al. Long-term islet allograft function in the absence of chronic immunosuppression: a case report of a nonhuman primate previously made tolerant to a renal allograft from the same donor. Transplantation 72, 351–354 (2001).
Wijkstrom, M., Kenyon, N. S. & Kirchhof, N. Islet allograft survival in nonhuman primates immunosuppressed with BasiliximabRAD, and FTY720. Amer. J. Transplantation (in the press).
Methods in Cell Transplantation (eds Ricordi, C. et al.) (RG Landes Co., Austin, Texas, 1995).
Bennet, W. et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes 48, 1907–1914 (1999).
Moberg, L. et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet 360, 2039–2045 (2002).
Mandrup-Poulsen, T., Bendtzen, K., Dinarello, C. A. & Nerup, J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic β-cell cytotoxicity. J. Immunol. 139, 4077–4082 (1987).
Kaufman, D. B. et al. Differential roles of Mac-1+ cells, and CD4+ and CD8+ T lymphocytes in primary nonfunction and classic rejection of islet allografts. J. Exp. Med. 172, 291–302 (1990).
Kaufman, D. B. et al. Effect of 15-deoxyspergualin on immediate function and long-term survival of transplanted islets in murine recipients of a marginal islet mass. Diabetes 43, 778–783 (1994).
Bottino, R. et al. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes 47, 316–323 (1998).
Bendtzen, K. et al. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science 232, 1545–1547 (1986).
Acknowledgements
This work was supported in part by grants from the National Institutes of Health, the Juvenile Diabetes Research Foundation Center for β-Cell Replacement and Tolerance Induction at the University of Miami-Diabetes Research Institute, the Juvenile Diabetes Research Foundation-Harvard Medical School Islet Transplant Center, the American Diabetes Association and the Diabetes Research Institute Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- HYPERGLYCAEMIA
-
Increased plasma-glucose concentrations that are above defined normal standards.
- NON-OBESE DIABETIC (NOD) MICE
-
A strain of mouse that normally develops idiopathic autoimmune diabetes through a disease process that closely resembles type 1 diabetes in humans. A series of pancreas islet-cell-specific target antigen(s) are recognized by pathogenic CD4+ T cells, but their identities have remained elusive.
- FK506
-
The initial name given to tacrolimus, a metabolite of the fungus Streptomyces tsukubaensis. It is a potent immunosuppressive agent that inhibits the formation of important growth-promoting cytokines, including interleukin-2.
- RAPAMYCIN
-
A new immunosuppressive drug that is structurally related to tacrolimus. It is produced by the organism Streptomyces hygroscopicus. It prevents the translation of mRNAs encoding cell-cycle regulators and controls progression from the G1 to S phase of the cell cycle.
- CENTRAL TOLERANCE
-
This form of tolerance refers to the lack of self-responsiveness found as lymphoid cells develop, and is associated with the deletion of autoreactive clones. For T cells, this occurs in the thymus. Many facets of classical central tolerance are evident in mixed donor and recipient haematopoietic chimaeras that are rendered tolerant to transplanted donor tissue allografts.
- PERIPHERAL TOLERANCE
-
Refers to the lack of responsiveness of mature lymphocytes to specific antigen. In transplant and autoimmune models, peripheral tolerance depends on active T-cell-dependent immunoregulation.
- ACTIVATION-INDUCED CELL DEATH
-
(AICD). The apoptotic cell death of activated lymphocytes. It ensures the rapid elimination of effector cells after their antigen-dependent clonal expansion. Defects in AICD result in lymphoproliferative diseases that are associated with autoimmune disorders.
- PASSIVE CELL DEATH
-
The death of T cells due to activation in the absence of sufficient survival signals, or when antigen is cleared and T-cell receptor signals cease.
- CYTOKINE TOXINS
-
Fusion proteins in which the cell-targeting sequences of diphtheria or pseudomonas exotoxin are replaced with a cytokine. The molecules are designed to target and intoxicate cells that express cytokine receptors.
Rights and permissions
About this article
Cite this article
Ricordi, C., Strom, T. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol 4, 259–268 (2004). https://doi.org/10.1038/nri1332
Issue Date:
DOI: https://doi.org/10.1038/nri1332
This article is cited by
-
Advances in alginate encapsulation of pancreatic islets for immunoprotection in type 1 diabetes
Journal of Pharmaceutical Investigation (2023)
-
Treg cell-based therapies: challenges and perspectives
Nature Reviews Immunology (2020)
-
Islet transplantation in the subcutaneous space achieves long-term euglycaemia in preclinical models of type 1 diabetes
Nature Metabolism (2020)
-
Translational Research Symposium—collaborative efforts as driving forces of healthcare innovation
Journal of Materials Science: Materials in Medicine (2019)
-
Immunosuppressive effect of arsenic trioxide on islet xenotransplantation prolongs xenograft survival in mice
Cell Death & Disease (2018)