Key Points
-
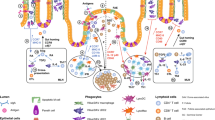
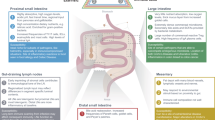
The intestinal immune system is an anatomically and functionally distinct compartment, in which a careful distinction must be made between harmful antigens, such as invasive pathogens, and harmless antigens, such as dietary proteins or commensal bacteria.
-
The default response to harmless antigens is the induction of tolerance. A breakdown in this physiological process can lead to disease.
-
Immune responses and tolerance in the gut are initiated in organized lymphoid organs, such as the Peyer's patches and mesenteric lymph nodes (MLNs). The mucosa contains effector or regulatory cells that migrate there selectively, from the MLNs, in the lymph and bloodstream under the control of α4β7 integrins and the chemokine receptor CCR9.
-
Pathogens might enter the intestinal immune system through M cells in the follicle-associated epithelium of the Peyer's patches, whereas soluble antigens might gain access predominantly through the normal epithelium that covers the villus mucosa.
-
Peyer's patches, lamina propria and MLNs contain unusual populations of dendritic cells (DCs), some of which are characterized by the production of interleukin-10 (IL-10) and which polarize T cells to an IL-4-, IL-10- and transforming growth factor-β (TGF-β)-producing 'regulatory' phenotype.
-
Genetically determined factors, together with luminal bacteria, might act on epithelial and stromal components of the intestinal mucosa to produce a local microenvironment that is dominated by the constitutive production of prostaglandin E2 (PGE2), TGF-β and IL-10. Under physiological conditions, this favours the differentiation of regulatory DCs and T cells, which leads to systemic tolerance and/or immunoglobulin-A production.
Abstract
The intestinal immune system has to discriminate between harmful and beneficial antigens. Although strong protective immunity is essential to prevent invasion by pathogens, equivalent responses against dietary proteins or commensal bacteria can lead to chronic disease. These responses are normally prevented by a complex interplay of regulatory mechanisms. This article reviews the unique aspects of the local microenvironment of the intestinal immune system and discuss how these promote the development of regulatory responses that ensure the maintenance of homeostasis in the gut.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mowat, A. McI. & Weiner, H. L. in Mucosal Immunology, 2nd edition (eds Ogra, P. L. et al.) 587–617 (Academic Press, San Diego, 1999).
Strobel, S. & Mowat, A. McI. Immune responses to dietary antigens: oral tolerance. Immunol. Today 19, 173–181 (1998).
Faria, A. M. C. & Weiner, H. L. Oral tolerance: mechanisms and therapeutic applications. Adv. Immunol. 73, 153–164 (1999).
Mowat, A. McI. & Viney, J. L. The anatomical basis of mucosal immune responses. Immunol. Rev. 156, 145–166 (1997).
Hamada, H. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
Mebius, R. E. Organogenesis of lymphoid tissue. Nature Rev. Immunol. 3, 292–303 (2003).
Alcamo, E. et al. Requirement for the NF-κB family member RelA in the development of secondary lymphoid organs. J. Exp. Med. 195, 233–244 (2002).
Honda, K. et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J. Exp. Med. 193, 621–630 (2001).
Browning, J. L. & French, L. E. Visualization of lymphotoxin-β and lymphotoxin-β receptor expression in mouse embryos. J. Immunol. 168, 5079–5087 (2002).
Yoshida, H. et al. Different cytokines induce surface lymphotoxin-αβ on IL7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity 17, 823–833 (2002).
Yilmaz, Z. B., Weih, D. S., Sivakumar, V. & Weih, F. RelB is required for Peyer's patch development: differential regulation of p52–RelB by lymphotoxin and TNF. EMBO J. 22, 121–130 (2003).
Debard, N., Sierro, F., Browning, J. & Kraehenbuhl, J. P. Effect of mature lymphocytes and lymphotoxin on the development of the follicle-associated epithelium and M cells in mouse Peyer's patches. Gastroenterology 120, 1173–1182 (2001).
Kernéis, S., Bogdanova, A., Kraehenbuhl, J. -P. & Pringault, E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 277, 949–952 (1997).
Golovkina, T. V., Shlomchik, M., Hannum, L. & Chervonsky, A. Organogenic role of B lymphocytes in mucosal immunity. Science 286, 1965–1968 (1999).
Debard, N., Sierro, F. & Kraehenbuhl, J. P. Development of Peyer's patches, follicle-associated epithelium and M cell: lessons from immunodeficient and knockout mice. Semin. Immunol. 11, 183–191 (1999).
Cuff, C. A., Sacca, R. & Ruddle, N. H. Differential induction of adhesion molecule and chemokine expression by LTα3 and LTαβ in inflammation elucidates potential mechanisms of mesenteric and peripheral lymph node development. J. Immunol. 162, 5965–5972 (1999).
Scheu, S. et al. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin β in mesenteric lymph node genesis. J. Exp. Med. 195, 1613–1624 (2002).
Wagner, N. et al. L-selectin and β7 integrin synergistically mediate lymphocyte migration to mesenteric lymph nodes. Eur. J. Immunol. 28, 3832–3839 (1998). This paper uses appropriate knockout and double-knockout mice to investigate the selective requirements for different adhesion molecules in the development of the gut-associated lymphoid tissues.
McIntyre, T. M. & Strober, W. in Mucosal Immunology, 2nd edition (eds Ogra, P. L. et al.) 319–356 (Academic Press, San Diego, 1999).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Butcher, E. C., Williams, M., Youngman, K., Rott, L. & Briskin, M. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72, 209–253 (1999).
Bowman, E. P. et al. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J. Exp. Med. 195, 269–275 (2002).
Campbell, D. J. & Butcher, E. C. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195, 135–141 (2002). This study provides a molecular basis for how the site of T-cell priming can determine their subsequent dissemination to peripheral or mucosal tissues.
Braunstein, J., Qiao, L., Autschbach, F., Schurmann, G. & Meuer, S. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut 41, 215–220 (1997).
Carol, M. et al. Spontaneous secretion of interferon γ and interleukin-4 by human intraepithelial and lamina propria gut lymphocytes. Gut 42, 603–604 (1998).
Hurst, S. D. et al. The differentiated state of intestinal lamina propria CD4+ T cells results in altered cytokine production, activation threshold, and costimulatory requirements. J. Immunol. 163, 5937–5945 (1999).
Lefrançois, L., Olson, S. & Masopust, D. A critical role for CD40–CD40 ligand interactions in amplification of the mucosal CD8 T cell response. J. Exp. Med. 190, 1275–1283 (1999).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Reinhardt, R. L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M. K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissues. Science 291, 2413–2417 (2001).
Khoo, U. Y., Proctor, I. E. & Macpherson, A. J. CD4+ T cell down-regulation in human intestinal mucosa: evidence for intestinal tolerance to luminal bacterial antigens. J. Immunol. 158, 3626–3634 (1997). This paper is one of the few to provide direct evidence that CD4+ T cells in the intestinal mucosa normally recognize the local commensal bacteria, but that their responses are inhibited by local regulatory T cells in an interleukin-10 (IL-10) and/or transforming growth factor-β (TGF-β)-mediated manner.
Read, S. & Powrie, F. CD4+ regulatory T cells. Curr. Opin. Immunol. 13, 644–649 (2001).
Shevach, E. M. CD4+ CD25+ suppressor T cells: more questions than answers. Nature Rev. Immunol. 2, 389–400 (2002).
Zimmer, K. P., Buning, J., Weber, P., Kaiserlian, D. & Strobel, S. Modulation of antigen trafficking to MHC class II-positive late endosomes of enterocytes. Gastroenterology 118, 128–137 (2000).
Hershberg, R. M. et al. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J. Clin. Invest. 102, 792–803 (1998).
Sanderson, I., Ouellette, A. J., Carter, E. A., Walker, W. A. & Harmatz, P. R. Differential regulation of B7 mRNA in enterocytes and lymphoid cells. Immunology 79, 434–438 (1993).
MacDonald, T. T. & Pender, S. L. F. Lamina propria T cells. Chem. Immunol. 71, 103–117 (1998).
Gütgemann, I., Fahrer, A. M., Davis, M. M. & Chien, Y. -H. Induction of rapid T-cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity 8, 667–673 (1998).
Williamson, E., O'Malley, J. M. & Viney, J. L. Visualizing the T-cell response elicited by oral administration of soluble protein antigen. Immunology 97, 565–572 (1999).
Van Houten, N. & Blake, S. F. Direct measurement of anergy of antigen-specific T cells following oral tolerance induction. J. Immunol. 157, 1337–1341 (1996).
Sun, J., Dirden-Kramer, B., Ito, K., Ernst, P. B. & Van Houten, N. Antigen-specific T cell activation and proliferation during oral tolerance induction. J. Immunol. 162, 5868–5875 (1999).
Smith, K. M., Davidson, J. M. & Garside, P. T-cell activation occurs simultaneously in local and peripheral lymphoid tissue following oral administration of a range of doses of immunogenic or tolerogenic antigen although tolerized T cells display a defect in cell division. Immunology 106, 144–158 (2002).
Karlsson, M. et al. 'Tolerosomes' are produced by intestinal epithelial cells. Eur. J. Immunol. 31, 2892–2900 (2001).
Thery, C. et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 166, 7309–7318 (2001).
Fujihashi, K. et al. Peyer's patches are required for oral tolerance to proteins. Proc. Natl Acad. Sci. USA 98, 3310–3315 (2001).
Spahn, T. W. et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur. J. Immunol. 31, 1278–1287 (2001).
Spahn, T. W. et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur. J. Immunol. 32, 1109–1113 (2002). Using genetic knockouts or treatment with a soluble receptor in utero that blocks different lymphotoxin (LT)-family members and their receptors, this paper shows that mesenteric lymph nodes, but not Peyer's patches, are required for the induction of various oral-tolerance regimes.
Alpan, O., Rudomen, G. & Matzinger, P. The role of dendritic cells, B cells and M cells in gut-oriented immune responses. J. Immunol. 166, 4843–4852 (2001). B-cell-deficient mice do not have normal Peyer's patches or M cells, but can be tolerized normally by feeding of protein antigen. This is associated with the presence of antigen-loaded dendritic cells in the mesenteric lymph nodes that induce T cells to produce IL-4 and IL-10.
Enders, G., Gottwald, T. & Brendel, W. Induction of oral tolerance in rats without Peyer's patches. Immunology 58, 311–314 (1986).
Kang, H. S. et al. Signalling via LTβR on the lamina propria stromal cells of the gut is required for IgA production. Nature Immunol. 3, 576–582 (2002). This study uses bone-marrow chimeras and the transplantation of gut from Rag-deficient mice to show that the defective IgA production in LTα-deficient mice is not necessarily related to abnormalities in the Peyer's patches. Rather, non-bone-marrow-derived cells in the lamina propria can provide the appropriate microenvironment for recruitment and differentiation of IgA precursors after activation through the LTβ receptor.
Kunisawa, J. et al. Lack of antigen-specific immune responses in anti-IL-7 receptor-α chain antibody-treated Peyer's patch-null mice following intestinal immunization with microencapsulated antigen. Eur. J. Immunol. 32, 2347–2355 (2002).
Yamamoto, M. et al. Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract. J. Immunol. 164, 5184–5191 (2000).
Chen, Y., Inobe, J. -I. & Weiner, H. L. Inductive events in oral tolerance in the TCR transgenic adoptive-transfer model. Cell. Immunol. 178, 62–68 (1997).
Meyer, A. L. et al. Rapid depletion of peripheral antigen-specific T cells in TCR-transgenic mice after oral administration of myelin basic protein. J. Immunol. 166, 5773–5782 (2001).
Shi, H. N., Liu, H. Y. & Nagler-Anderson, C. Enteric infection as an adjuvant for the response to a model food antigen. J. Immunol. 165, 6174–6182 (2000).
Blanas, E., Carbone, F. R. & Heath, W. R. A bone marrow-derived APC in the gut-associated lymphoid tissue captures oral antigens and presents them to both CD4+ and CD8+ T cells. J. Immunol. 164, 2890–2896 (2000).
Lee, H. O. et al. Interferon-γ induction during oral tolerance reduces T-cell migration to sites of inflammation. Gastroenterology 119, 129–138 (2000).
Lefrancois, L., Altman, J. D., Williams, K. & Olson, S. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J. Immunol. 164, 725–732 (2000).
MacPherson, G. G. & Liu, L. M. Dendritic cells and Langerhans cells in the uptake of mucosal antigens. Curr. Top. Microbiol. Immunol. 236, 33–53 (1999).
Huang, F. P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000).
Ruedl, C., Rieser, C., Bock, G., Wick, G. & Wolf, H. Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer's patches. Eur. J. Immunol. 26, 1801–1806 (1996).
Ruedl, C. & Hubele, S. Maturation of Peyer's patch dendritic cells in vitro upon stimulation via cytokines or CD40 triggering. Eur. J. Immunol. 27, 1325–1330 (1997).
Iwasaki, A. & Kelsall, B. L. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin-10 and induce the differentiation of T-helper type 2 cells. J. Exp. Med. 190, 229–239 (1999).
Iwasaki, A. & Kelsall, B. L. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191, 1381–1393 (2000).
Iwasaki, A. & Kelsall, B. L. Unique functions of CD11b+, CD8α+ and double negative Peyer's patch dendritic cells. J. Immunol. 166, 4884–4890 (2001). The description of individual and unique subsets of dendritic cells in mouse Peyer's patches, each with distinct functions, which include the preferential stimulation of IL-4- and IL-10-producing T cells.
Cook, D. N. et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12, 495–503 (2000).
Williamson, E., Bilsborough, J. M. & Viney, J. L. Regulation of mucosal dendritic cell function by receptor activator of NF-κB (RANK)/RANK ligand interactions: impact on tolerance induction. J. Immunol. 169, 3606–3612 (2002).
Stumbles, P. A. et al. Resting respiratory tract dendritic cells preferentially stimulate helper cell type 2 (TH2) responses and require obligatory cytokine signals for induction of TH1 immunity. J. Exp. Med. 188, 2019–2031 (1998).
Akbari, O., DeKruyff, R. H. & Umetsu, D. T. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nature Immunol. 2, 725–731 (2001). By purifying dendritic cells (DCs) from the lymphoid tissues that drain from the respiratory tract and gut, this paper shows that intranasal antigen associates with a population of IL-10-producing DCs that can induce IL-10-dependent T-cell tolerance in vivo . Parallel studies indicate that after feeding antigen, antigen-loaded DCs in the mesenteric lymph nodes produce TGF-β and stimulate CD4+ T cells to produce both IL-10 and TGF-β.
Martin, P. et al. Characterization of a new subpopulation of mouse CD8α+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood 100, 383–390 (2002).
Bell, S. J. et al. Migration and maturation of human colonic dendritic cells. J. Immunol. 166, 4958–4967 (2001).
Harper, H., Cochrane, L. & Williams, N. A. The role of small intestinal antigen-presenting cells in the induction of T-cell reactivity to soluble protein antigens: association between aberrant presentation in the lamina propria and oral tolerance. Immunology 89, 449–456 (1996).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2, 361–367 (2001). An in vitro co-culture model showing that DCs normally do not invade monolayers of intestinal epithelial cells. However, if bacteria are placed on the apical surface of the enterocytes, DCs are stimulated to invade the epithelium and might even form tight junctions with neighbouring enterocytes to preserve the barrier. Some of the DCs can take up bacteria from the surface of the epithelium before withdrawing from the monolayer. A similar phenomenon might occur in vivo.
Anjuere, F. et al. Definition of dendritic cell subpopulations present in the spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood 93, 590–598 (1999).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997).
Stagg, A. J., Kamm, M. A. & Knight, S. C. Intestinal dendritic cells increase T-cell expression of α4β7 integrin. Eur. J. Immunol. 32, 1445–1454 (2002).
Thomson, A. W., O'Connell, P. J., Steptoe, R. J. & Lu, L. Immunobiology of liver dendritic cells. Immunol. Cell. Biol. 80, 65–73 (2002).
Mehal, W. Z., Azzaroli, F. & Crispe, I. N. Immunology of the healthy liver: old questions and new insights. Gastroenterology 120, 250–260 (2001).
Gorczynski, R. M., Cohen, Z., Levy, G. & Fu, X. M. A role for γδTCR+ cells in regulation of rejection of small intestinal allografts in rats. Transplantation 62, 844–851 (1996).
Mowat, A. McI. in The Immunology of the Liver (ed. Crispe, I. N.) 101–115 (John Wiley & Sons Inc., New York, 1999).
Yang, R., Liu, Q., Grosfeld, J. L. & Pescovitz, M. D. Intestinal drainage through liver is a pre-requisite for oral tolerance induction. J. Pediatr. Surg. 29, 1145–1148 (1994).
Thomas, H. C., Ryan, C. J., Benjamin, I. S., Blumgart, L. H. & MacSween, R. N. M. The immune system response in cirrhotic rats. The induction of tolerance to orally administered protein antigens. Gastroenterology 71, 114–117 (1976).
Premier, R. R. & Meeusen, E. N. T. Lymphocyte surface marker and cytokine expression in peripheral and mucosal lymph nodes. Immunology 94, 363–367 (1998).
Kellermann, S. A. & McEvoy, L. M. The Peyer's patch microenvironment suppresses T cell responses to chemokines and other stimuli. J. Immunol. 167, 682–690 (2001).
Jump, R. L. & Levine, A. D. Murine Peyer's patches favor development of an IL-10-secreting, regulatory T cell population. J. Immunol. 168, 6113–6119 (2002).
Wolvers, D. A. W. et al. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp39. J. Immunol. 162, 1994–1998 (1999). An intriguing study that shows that surgical removal of the draining lymph nodes abrogates the induction of tolerance by intranasal administration of proteins and that only the appropriate lymph node can restore tolerance induction. So, lymph nodes that drain from mucosal tissues might be functionally distinct from other lymph nodes.
Neish, A. S. et al. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science 289, 1560–1563 (2000). This paper shows that commensal bacteria in the gut are not merely passive spectators in the local microenvironment, as they can interact with enterocytes and actively inhibit the inflammatory signalling processes that are stimulated by pathogenic organisms. The implication is that commensal bacteria are continually involved in signalling to enterocytes and this might be important for the maintenance of normal homeostasis.
Haller, D. et al. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 47, 79–87 (2000).
Fagarasan, S., Kinoshita, K., Muramatsu, M., Ikuta, K. & Honjo, T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature 413, 639–643 (2001).
Newberry, R. D., Stenson, W. P. & Lorenz, R. G. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nature Med. 5, 900–906 (1999). This is the only study to have reported an animal model in which pathology similar to coeliac disease can be induced by feeding antigen. This requires blocking of a constitutive pathway of prostaglandin (PG)E 2 production, and the authors suggest that this reflects a physiological process by which local bacteria normally promote the downregulation of local immune responses. This would involve the stimulation of PGE 2 by stromal cells, which then induces the differentiation of IL-10-producing DCs.
Newberry, R. D., McDonough, J. S., Stenson, W. F. & Lorenz, R. G. Spontaneous and continuous cyclooxygenase-2-dependent prostaglandin E2 production by stromal cells in the murine small intestinal lamina propria: directing the tone of the intestinal immune response. J. Immunol. 166, 4465–4472 (2001).
Harizi, H., Juzan, M., Pitard, V., Moreau, J. F. & Gualde, N. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic-cell functions. J. Immunol. 168, 2255–2263 (2002).
Kalinski, P., Schuitemaker, J. H., Hilkens, C. M. & Kapsenberg, M. L. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 161, 2804–2809 (1998).
Williamson, E., Westrich, G. M. & Viney, J. L. Modulating dendritic cells to optimize mucosal immunization protocols. J. Immunol. 163, 3668–3675 (1999).
MacPherson, G. G., Jenkins, C. D., Stein, M. J. & Edwards, C. Endotoxin-mediated dendritic cell release from the intestine. Characterization of released dendritic cells and TNF dependence. J. Immunol. 154, 1317–1322 (1995).
Miller, A., Lider, O., Roberts, A. B., Sporn, M. B. & Weiner, H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor β after antigen-specific triggering. Proc. Natl Acad. Sci. USA 89, 421–425 (1992).
Thorstenson, K. M. & Khoruts, A. Generation of anergic and potentially immunoregulatory CD25+CD4+ T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167, 188–195 (2001).
Mowat, A. M. The regulation of immune responses to dietary protein antigens. Immunol. Today 8, 93–98 (1987).
Ke, Y., Pearce, K., Lake, J. P., Ziegler, H. K. & Kapp, J. A. γδ T lymphocytes regulate the induction of oral tolerance. J. Immunol. 158, 3610–3618 (1997).
Acknowledgements
I would like to thank L. Cousins for critical review of the manuscript.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
OMIM
Glossary
- COELIAC DISEASE
-
A chronic inflammatory condition of the upper small intestine in humans that is caused by immunological hypersensitivity to the α-gliadin component of wheat gluten. It is often found in infants after the introduction of solid foods. It causes severe villus atrophy, which can lead to malabsorption and malnutrition if gluten-containing foods are not removed from the diet.
- CROHN'S DISEASE
-
A form of chronic inflammatory bowel disease that can affect the entire gastrointestinal tract, but is commonest in the colon and terminal ileum. It is characterized by transmural inflammation, strictures and granuloma formation, and is believed to result from an abnormal T-cell-mediated immune response to commensal bacteria.
- BRUSH BORDER
-
The surface layer of the normal small intestine that is comprised of small microvilli coated in a rich glycocalyx of mucus and other glycoproteins. The microvilli contain many of the digestive enzymes and transporter systems that are involved in the metabolism and uptake of dietary materials. The brush border provides a large surface area for absorption.
- INFLAMMATORY BOWEL DISEASE
-
A chronic condition of the intestine that is characterized by severe inflammation and mucosal destruction. The commonest forms in humans are ulcerative colitis and Crohn's disease. Animal models indicate that they result from the dysregulation of the local immune response to normally harmless commensal bacteria.
- EXOSOMES
-
Small lipid-bilayer vesicles that are released from dendritic cells and other cells. They are comprised of either cell membranes or the membranes of intracellular vesicles. They might contain antigen–MHC complexes and interact with antigen-specific lymphocytes directly, or they might be taken up by further antigen-presenting cells.
- PLASMACYTOID DENDRITIC CELLS
-
A subset of dendritic cells (DCs) with a microscopic appearance similar to plasmablasts. In humans, these DCs are the main producers of type I interferon (IFN) in response to virus infections. Recent studies have identified a similar subset of type I IFN-producing DCs in mice, which are characterized by expression of B220 and Ly6C/G, and which might be tolergenic in nature.
- DANGER SIGNALS
-
Cell-wall components and other products of pathogens that alert the innate immune system to the presence of potentially harmful invaders, usually by interacting with Toll-like receptors and other pattern-recognition receptors expressed by tissue cells and dendritic cells, for example.
Rights and permissions
About this article
Cite this article
Mowat, A. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 3, 331–341 (2003). https://doi.org/10.1038/nri1057
Issue Date:
DOI: https://doi.org/10.1038/nri1057
This article is cited by
-
Deoxynivalenol triggers the expression of IL-8-related signaling cascades and decreases protein biosynthesis in primary monocyte-derived cells
Mycotoxin Research (2024)
-
Immunomodulatory effects of different strains of Lactococcus lactis in DSS-induced colitis
Brazilian Journal of Microbiology (2023)
-
Mechanistic Insights into Immune-Microbiota Interactions and Preventive Role of Probiotics Against Autoimmune Diabetes Mellitus
Probiotics and Antimicrobial Proteins (2023)
-
Issues for patchy tissues: defining roles for gut-associated lymphoid tissue in neurodevelopment and disease
Journal of Neural Transmission (2023)
-
Evaluating Salmonella pullorum dissemination and shedding patterns and antibody production in infected chickens
BMC Veterinary Research (2022)