Key Points
-
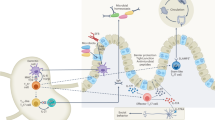
In addition to their well-characterized role in immune pathology, T helper 17 (TH17) cells maintain homeostasis at barrier sites by containing commensal bacteria, inducing the expression of tight junction proteins and antimicrobial peptides, and fighting pathogens.
-
Segmented filamentous bacteria (SFB) are the main drivers of the differentiation of intestinal TH17 cells, which in turn control SFB burden through interleukin-17 (IL-17) signalling in intestinal epithelial cells.
-
The substantial degree of plasticity displayed by TH17 cells is context-dependent and can be beneficial (for example, having an intestinal regulatory function or promoting IgA production) or pathogenic (for example, inducing the production of additional pro-inflammatory cytokines during central nervous system inflammation).
-
A multitude of environmental factors — including diet, aryl hydrocarbon receptor ligands, circadian rhythms and the microbiota — influence the function of TH17 cells.
-
When targeting TH17 cells in inflammatory diseases, we need to ensure that the protective functions of intestinal TH17 cells are not compromised.
Abstract
T helper 17 (TH17) cells have been extensively studied since their discovery 10 years ago, primarily because of their known pathogenic role in many inflammatory diseases. Substantial progress has been made in understanding their development, regulation and functional activities, and genome-wide transcriptomic analysis has identified regulatory networks, nodes and interactions that provide vital clues for further studies. In this Review, we describe recent studies that have revealed the dichotomous nature of TH17 cells, which on the one hand allows these cells to be pathogenic drivers of inflammatory disorders and on the other hand allows them to support the integrity of the intestinal barrier in a non-inflammatory manner.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hernandez-Santos, N. et al. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 6, 900–910 (2013).
Chen, K. & Kolls, J. K. T cell-mediated host immune defenses in the lung. Annu. Rev. Immunol. 31, 605–633 (2013).
Yosef, N. et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature 496, 461–468 (2013).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012). This article provides a comprehensive overview of the transcription factors and regulators that are involved in T H 17 cell differentiation.
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012).
Gaublomme, J. T. et al. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 163, 1400–1412 (2015). In this study, single-cell RNA-sequencing analysis enables the identification of factors that determine T H 17 cell heterogeneity and pathogenicity.
Hirota, K. et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12, 255–263 (2011). This study describes the first IL-17 fate reporter and demonstrates extensive T H cell plasticity in EAE, showing that many pro-inflammatory cytokines other than IL-17 are derived from cells with a T H 17 cell origin.
Tuomela, S. et al. Comparative analysis of human and mouse transcriptomes of Th17 cell priming. Oncotarget 7, 13416–13428 (2016).
McGeachy, M. J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
Ghoreschi, K. et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Veldhoen, M., Hocking, R. J., Flavell, R. A. & Stockinger, B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 7, 1151–1156 (2006).
Heink, S. et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat. Immunol. 18, 74–85 (2017).
Wang, C. et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163, 1413–1427 (2015).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Meyer Zu Horste, G. et al. RBPJ controls development of pathogenic Th17 cells by regulating IL-23 receptor expression. Cell Rep. 16, 392–404 (2016).
Kishi, Y. et al. Protein C receptor (PROCR) is a negative regulator of Th17 pathogenicity. J. Exp. Med. 213, 2489–2501 (2016).
Ichiyama, K. et al. The microRNA-183-96-182 cluster promotes T helper 17 cell pathogenicity by negatively regulating transcription factor Foxo1 expression. Immunity 44, 1284–1298 (2016).
Du, C. et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat. Immunol. 10, 1252–1259 (2009).
Sutton, C., Brereton, C., Keogh, B., Mills, K. H. & Lavelle, E. C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203, 1685–1691 (2006).
Murugaiyan, G. et al. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J. Clin. Invest. 125, 1069–1080 (2015).
Mycko, M. P. et al. MicroRNA-301a regulation of a T-helper 17 immune response controls autoimmune demyelination. Proc. Natl Acad. Sci. USA 109, E1248–E1257 (2012).
Escobar, T. M. et al. miR-155 activates cytokine gene expression in Th17 cells by regulating the DNA-binding protein Jarid2 to relieve Polycomb-mediated repression. Immunity 40, 865–879 (2014).
Sandhu, S. K. et al. miR-155 targets histone deacetylase 4 (HDAC4) and impairs transcriptional activity of B-cell lymphoma 6 (BCL6) in the Eμ-miR-155 transgenic mouse model. Proc. Natl Acad. Sci. USA 109, 20047–20052 (2012).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Huang, W. et al. DDX5 and its associated lncRNA Rmrp modulate TH17 cell effector functions. Nature 528, 517–522 (2015).
Kofler, D. M. et al. Decreased RORC-dependent silencing of prostaglandin receptor EP2 induces autoimmune Th17 cells. J. Clin. Invest. 124, 2513–2522 (2014).
Zielinski, C. E. et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012).
Becattini, S. et al. T cell immunity. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science 347, 400–406 (2015).
Harbour, S. N., Maynard, C. L., Zindl, C. L., Schoeb, T. R. & Weaver, C. T. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl Acad. Sci. USA 112, 7061–7066 (2015).
Morrison, P. J. et al. Th17-cell plasticity in Helicobacter hepaticus-induced intestinal inflammation. Mucosal Immunol. 6, 1143–1156 (2013).
Ahlfors, H. et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J. Immunol. 193, 4602–4613 (2014).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Krebs, C. F. et al. Plasticity of Th17 cells in autoimmune kidney diseases. J. Immunol. 197, 449–457 (2016).
Krebs, C. F. et al. Autoimmune renal disease is exacerbated by S1P-receptor-1-dependent intestinal Th17 cell migration to the kidney. Immunity 45, 1078–1092 (2016).
Jain, R. et al. Interleukin-23-induced transcription factor Blimp-1 promotes pathogenicity of T helper 17 cells. Immunity 44, 131–142 (2016).
Krausgruber, T. et al. T-Bet is a key modulator of IL-23-driven pathogenic CD4+ T cell responses in the intestine. Nat. Commun. 7, 11627 (2016). This study identifies T-bet as an important regulator of the IL-23-driven pathogenicity of T H 17 cells.
Ahern, P. P., Izcue, A., Maloy, K. J. & Powrie, F. The interleukin-23 axis in intestinal inflammation. Immunol. Rev. 226, 147–159 (2008).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Hirota, K. et al. Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 14, 372–379 (2013).
Gagliani, N. et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 (2015).
Cua, D. J. & Tato, C. M. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 (2010).
Conti, H. R. et al. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J. Exp. Med. 211, 2075–2084 (2014).
Puel, A. et al. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr. Opin. Allergy Clin. Immunol. 12, 616–622 (2012).
Busbee, P. B., Rouse, M., Nagarkatti, M. & Nagarkatti, P. S. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev. 71, 353–369 (2013).
Nguyen, L. P. & Bradfield, C. A. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 21, 102–116 (2008).
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Stockinger, B., Di Meglio, P., Gialitakis, M. & Duarte, J. H. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 32, 403–432 (2014).
Endo, Y. et al. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, ACC1. Cell Rep. 12, 1042–1055 (2015).
Berod, L. et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20, 1327–1333 (2014).
Garidou, L. et al. The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease. Cell Metab. 22, 100–112 (2015).
Kleinewietfeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013).
Wu, C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013).
Yu, X. et al. TH17 cell differentiation is regulated by the circadian clock. Science 342, 727–730 (2013).
Farez, M. F. et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162, 1338–1352 (2015).
Cummins, E. P. & Crean, D. Hypoxia and inflammatory bowel disease. Microbes Infect. 19, 210–221 (2017).
Glover, L. E., Lee, J. S. & Colgan, S. P. Oxygen metabolism and barrier regulation in the intestinal mucosa. J. Clin. Invest. 126, 3680–3688 (2016).
Dang, E. V. et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Wang, H. et al. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat. Immunol. 15, 393–401 (2014).
Kelly, C. J. et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671 (2015).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Prakash, T. et al. Complete genome sequences of rat and mouse segmented filamentous bacteria, a potent inducer of Th17 cell differentiation. Cell Host Microbe 10, 273–284 (2011).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Schnupf, P., Gaboriau-Routhiau, V. & Cerf-Bensussan, N. Host interactions with segmented filamentous bacteria: an unusual trade-off that drives the post-natal maturation of the gut immune system. Semin. Immunol. 25, 342–351 (2013).
Kumar, P. et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity 44, 659–671 (2016).
Yang, Y. et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 510, 152–156 (2014). This study provides evidence that intestinal T H 17 cells have TCR specificity for SFB.
Panea, C. et al. Intestinal monocyte-derived macrophages control commensal-specific Th17 responses. Cell Rep. 12, 1314–1324 (2015).
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40, 594–607 (2014).
Sano, T. et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 163, 381–393 (2015). This study identifies the mechanisms by which the interaction between SFB and intestinal epithelial cells leads to the induction of T H 17 cells.
Atarashi, K. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015). This paper shows that T H 17 cell induction in the gut is shaped by microorganisms that attach to intestinal epithelial cells.
Yin, Y. et al. Comparative analysis of the distribution of segmented filamentous bacteria in humans, mice and chickens. ISME J. 7, 615–621 (2013).
Torchinsky, M. B., Garaude, J., Martin, A. P. & Blander, J. M. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature 458, 78–82 (2009).
Sansonetti, P. J. To be or not to be a pathogen: that is the mucosally relevant question. Mucosal Immunol. 4, 8–14 (2011).
Stepankova, R. et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm. Bowel Dis. 13, 1202–1211 (2007).
Wu, H. J. et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010).
Lee, Y. K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4615–4622 (2011).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Kusu, T. et al. Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J. Immunol. 190, 774–783 (2013).
Kolls, J. K., McCray, P. B. Jr & Chan, Y. R. Cytokine-mediated regulation of antimicrobial proteins. Nat. Rev. Immunol. 8, 829–835 (2008).
Kinugasa, T., Sakaguchi, T., Gu, X. & Reinecker, H. C. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 118, 1001–1011 (2000).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738 (2015).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Birchenough, G. M., Johansson, M. E., Gustafsson, J. K., Bergstrom, J. H. & Hansson, G. C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 8, 712–719 (2015).
Basu, R. et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37, 1061–1075 (2012).
Behnsen, J. et al. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 40, 262–273 (2014).
Patel, D. D. & Kuchroo, V. K. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity 43, 1040–1051 (2015).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+CD3+CD4−CD8− entheseal resident T cells. Nat. Med. 18, 1069–1076 (2012).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Colombel, J. F., Sendid, B., Jouault, T. & Poulain, D. Secukinumab failure in Crohn's disease: the yeast connection? Gut 62, 800–801 (2013).
Puel, A. et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332, 65–68 (2011).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N. Engl. J. Med. 375, 1946–1960 (2016).
Xiao, S. et al. Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 40, 477–489 (2014).
Withers, D. R. et al. Transient inhibition of ROR-γt therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nat. Med. 22, 319–323 (2016).
Lim, H. W. et al. SIRT1 deacetylates RORγt and enhances Th17 cell generation. J. Exp. Med. 212, 607–617 (2015).
Ueda, E. et al. High incidence of T-cell lymphomas in mice deficient in the retinoid-related orphan receptor RORγ. Cancer Res. 62, 901–909 (2002).
Conti, H. R. & Gaffen, S. L. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J. Immunol. 195, 780–788 (2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Inflammatory bowel disease
-
(IBD). A chronic inflammatory condition that affects the intestinal tract. The proposed pathogenesis of IBD involves a complex model that includes functional abnormalities of innate immune cells and their relationship with the commensal microbiota; the inappropriate release of pro-inflammatory cytokines and other mediators; alterations of the intestinal epithelial barrier; and a cytokine imbalance that promotes the pro-inflammatory activity of adaptive immune cells.
- MicroRNAs
-
(miRNAs). Small RNA molecules that regulate the expression of genes by binding to the 3′ untranslated regions of specific mRNAs.
- Cell fate reporter mice
-
Mice in which the mapping of cell fates is based on two genetically engineered alleles that express a site-specific recombinase and a reporter allele that permanently expresses a marker following site-specific recombination. Cells that express the reporter are permanently marked with the fluorescent protein irrespective of subsequent changes to expression of the Cre recombinase-targeted gene.
- Transfer model of colitis
-
A mouse model of T cell-mediated pan-colitis and small bowel inflammation that is induced by the adoptive transfer of naive CD4+ T cells into syngeneic recipients that lack T cells and B cells.
- T follicular helper cells
-
(TFH cells). CD4+ T cells that provide help to B cells in follicles and germinal centres. The TFH cell signature includes the expression of CXC-chemokine receptor 5, inducible T cell co-stimulator, CD40 ligand and interleukin-21, which mediate TFH cell homing to follicles and B cell help.
- Peyer's patches
-
Groups of lymphoid nodules present in the small intestine that are massed together on the intestinal wall, opposite to the line of attachment of the mesentery. Peyer's patches consist of a subepithelial dome area, B cell follicles and interfollicular T cell zones.
- Activation-induced cytidine deaminase
-
An enzyme that is required for two crucial events in the germinal centre: somatic hypermutation and class-switch recombination.
- T regulatory type 1-like cells
-
(TR1-like cells). A subset of CD4+ regulatory T cells that secrete high levels of interleukin-10 (IL-10), and downregulate T helper 1 (TH1) and TH2 cell responses in vitro and in vivo by a contact-independent mechanism that is mediated by the secretion of soluble IL-10 and transforming growth factor-β.
- Group 3 innate lymphoid cells
-
(ILC3s). A subset of innate lymphoid cells that express RORγt. They can be further subdivided into CC-chemokine receptor 6 (CCR6)-expressing ILC3s, which are present at birth and produce both interleukin-17 (IL-17) and IL-22, and postnatal CCR6− ILC3s, which produce only IL-22. ILC3s are required for the development of intestinal cryptopatches and isolated lymphoid follicles, and are crucial for the maintenance of mucosal barriers, as they are the main source of homeostatic IL-22.
- Dysbiosis
-
A condition in which the balance of the bacterial communities that constitute the intestinal microbiota is altered; this condition could represent a predisposing factor for several diseases.
- Dextran sodium sulfate
-
(DSS). A large polysaccharide that causes epithelial injury and inflammation in the intestinal tract, and is commonly used in models of experimentally induced colitis, which are used to study the response to intestinal injury.
- Physiological hypoxia
-
A setting that does not result in any pathology and in which the partial oxygen pressure is decreased owing to organ-specific rates of vascularization.
- Tight junction
-
A belt-like region of adhesion between adjacent epithelial or endothelial cells that regulates paracellular flux. Tight junction proteins include the integral membrane proteins occludin and claudin, which associate with cytoplasmic zonula occludens proteins.
Rights and permissions
About this article
Cite this article
Stockinger, B., Omenetti, S. The dichotomous nature of T helper 17 cells. Nat Rev Immunol 17, 535–544 (2017). https://doi.org/10.1038/nri.2017.50
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2017.50
This article is cited by
-
Targeting tumor microenvironment with antibody-guided IL-2 pro-cytokine promotes and rejuvenates dysfunctional CD8+ T cells
Signal Transduction and Targeted Therapy (2023)
-
Cholinergic control of Th17 cell pathogenicity in experimental autoimmune encephalomyelitis
Cell Death & Differentiation (2023)
-
IL-1α-releasing TH17 cells live long and prosper
Nature Immunology (2023)
-
RORγt-Raftlin1 complex regulates the pathogenicity of Th17 cells and colonic inflammation
Nature Communications (2023)
-
TH17 cell heterogeneity and its role in tissue inflammation
Nature Immunology (2023)