Key Points
-
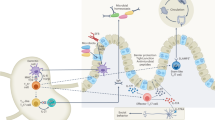
T helper 2 (TH2) cells respond to a variety of environmental cues, either directly or indirectly through interaction with cells of the innate immune system. For instance, certain specialized dendritic cells (DCs) promote TH2 cell induction, whereas other DC subsets are suppressive.
-
Epithelial cell-derived cytokines, such as IL-25, IL-33 and thymic stromal lymphopoietin (TSLP), and IL-4-producing immune cells, such as innate lymphoid cells and basophils, can potentiate TH2 cell responses. However, the relative importance of these innate cell stimuli for TH2 cell development remains to be determined and is likely to be dependent on local environmental cues.
-
TH2 cell differentiation is fundamentally dependent on the mechanistic target of rapamycin-mediated metabolic transition from oxidative phosphorylation to aerobic glycolysis.
-
TH cell subsets are somewhat heterogeneous in terms of their cytokine secretion and transcription factor profiles. Single-cell technologies promise to deliver new insight into how TH cells integrate diverse environmental cues to ensure their adaptability during homeostasis, protective immunity and tissue repair.
-
Our evolving knowledge of TH2 cell differentiation at the molecular and cellular levels has led to the development of novel therapies targeting specific transcription factors and TH2 cell-associated cytokines.
Abstract
T helper 2 (TH2) cells orchestrate protective type 2 immune responses, such as those that target helminths and facilitate tissue repair, but also contribute to chronic inflammatory diseases, such as asthma and allergy. Here, we review recent insights into how diverse molecular signals from cellular sources, including dendritic cells, innate lymphoid cells and the epithelium, are integrated by T cells to guide the transcriptional and epigenetic changes necessary for TH2 cell differentiation. Our improved understanding of these pathways has opened new avenues for therapeutically targeting TH2 cells in asthma and allergy. The advent of comprehensive single-cell transcriptomics along with improvements in single-cell proteomics and the generation of novel in vivo cell fate mapping techniques promise to expand our understanding of T cell diversity and offer new insight into disease-related heterogeneity and plasticity of TH cell responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mosmann, T. R., Cherwinski, H., Bond, M. W., Giedlin, M. A. & Coffman, R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357 (1986). This is a key paper describing the subsets of T H cells according to their cytokine production profiles.
Kelso, A. Th1 and Th2 subsets: paradigms lost? Immunol. Today 16, 374–379 (1995).
Shih, H. Y. et al. Transcriptional and epigenetic networks of helper T and innate lymphoid cells. Immunol. Rev. 261, 23–49 (2014).
Zhu, J. & Paul, W. E. CD4 T cells: fates, functions, and faults. Blood 112, 1557–1569 (2008).
Fallon, P. G. et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17, 7–17 (2002).
Paul, W. E. & Zhu, J. How are TH2-type immune responses initiated and amplified? Nat. Rev. Immunol. 10, 225–235 (2010).
MacDonald, A. S. & Maizels, R. M. Alarming dendritic cells for Th2 induction. J. Exp. Med. 205, 13–17 (2008).
van Rijt, L. S. et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201, 981–991 (2005).
Phythian-Adams, A. T. et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J. Exp. Med. 207, 2089–2096 (2010).
Radtke, F., MacDonald, H. R. & Tacchini-Cottier, F. Regulation of innate and adaptive immunity by Notch. Nat. Rev. Immunol. 13, 427–437 (2013).
Webb, G. J., Hirschfield, G. M. & Lane, P. J. OX40, OX40L and autoimmunity: a comprehensive review. Clin. Rev. Allergy Immunol. 50, 312–332 (2016).
Jenkins, S. J., Perona-Wright, G., Worsley, A. G., Ishii, N. & MacDonald, A. S. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J. Immunol. 179, 3515–3523 (2007).
Tindemans, I., Peeters, M. J. W. & Hendriks, R. W. Notch signaling in T helper cell subsets: instructor or unbiased amplifier? Front. Immunol. 8, 419 (2017).
Tindemans, I. et al. Notch signaling in T cells is essential for allergic airway inflammation, but expression of the Notch ligands Jagged 1 and Jagged 2 on dendritic cells is dispensable. J. Allergy Clin. Immunol. 140, 1079–1089 (2017).
Kumamoto, Y. et al. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 39, 733–743 (2013).
Connor, L. M., Tang, S. C., Camberis, M., Le Gros, G. & Ronchese, F. Helminth-conditioned dendritic cells prime CD4+ T cells to IL-4 production in vivo. J. Immunol. 193, 2709–2717 (2014).
Ito, T. et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202, 1213–1223 (2005).
Besnard, A. G. et al. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur. J. Immunol. 41, 1675–1686 (2011).
Chu, D. K. et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J. Allergy Clin. Immunol. 131, 187–200.e8 (2013).
Barrett, N. A., Maekawa, A., Rahman, O. M., Austen, K. F. & Kanaoka, Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J. Immunol. 182, 1119–1128 (2009).
Kim, D. C. et al. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J. Immunol. 176, 4440–4448 (2006).
Everts, B. et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J. Exp. Med. 209, 1753–1767, (2012).
Pulendran, B. et al. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167, 5067–5076 (2001).
Williams, J. W. et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 4, 2990 (2013).
Gao, Y. et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity 39, 722–732 (2013).
Tussiwand, R. et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 42, 916–928 (2015). This paper finds that KLF4 dependence identifies a subset of IRF4-dependent DCs that promotes T H 2 cell differentiation.
Satpathy, A. T. et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat. Immunol. 14, 937–948 (2013).
Leon, B. et al. Regulation of TH2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat. Immunol. 13, 681–690 (2012).
Halim, T. Y. et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat. Immunol. 17, 57–64 (2016).
Ulges, A. et al. Protein kinase CK2 enables regulatory T cells to suppress excessive TH2 responses in vivo. Nat. Immunol. 16, 267–275 (2015).
Lloyd, C. M. & Marsland, B. J. Lung homeostasis: influence of age, microbes, and the immune system. Immunity 46, 549–561 (2017).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014).
Everts, B. et al. Migratory CD103+ dendritic cells suppress helminth-driven type 2 immunity through constitutive expression of IL-12. J. Exp. Med. 213, 35–51 (2016).
Carrera Silva, E. A. et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity 39, 160–170 (2013).
Chan, P. Y. et al. The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science 352, 99–103 (2016).
Sokol, C. L., Barton, G. M., Farr, A. G. & Medzhitov, R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 9, 310–318 (2008).
Kim, S. et al. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J. Immunol. 184, 1143–1147 (2010).
Otsuka, A. et al. Basophils are required for the induction of Th2 immunity to haptens and peptide antigens. Nat. Commun. 4, 1739 (2013).
Perrigoue, J. G. et al. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat. Immunol. 10, 697–705 (2009).
Sokol, C. L. et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 10, 713–720 (2009).
Yoshimoto, T. et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat. Immunol. 10, 706–712 (2009).
Miyake, K. et al. Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc. Natl. Acad. Sci. USA 114, 1111–1116 (2017).
Eckl-Dorna, J. et al. Basophils are not the key antigen-presenting cells in allergic patients. Allergy 67, 601–608 (2012).
Kitzmüller, C. et al. Human blood basophils do not act as antigen-presenting cells for the major birch pollen allergen Bet v 1. Allergy 67, 593–600 (2012).
Sharma, M. et al. Circulating human basophils lack the features of professional antigen presenting cells. Sci. Rep. 3, 1188 (2013).
Hammad, H. et al. Inflammatory dendritic cells — not basophils — are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 207, 2097–2111 (2010).
Ohnmacht, C. et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33, 364–374 (2010).
Eberl, G., Colonna, M., Di Santo, J. P. & McKenzie, A. N. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566 (2015).
McKenzie, A. N., Spits, H. & Eberl, G. Innate lymphoid cells in inflammation and immunity. Immunity 41, 366–374 (2014).
Klose, C. S. & Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 17, 765–774 (2016).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010). This paper defines an alternative lymphocyte source of type 2 cytokines during helminth infection.
Oliphant, C. J. et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41, 283–295 (2014).
Mirchandani, A. S. et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J. Immunol. 192, 2442–2448 (2014).
Halim, T. Y. et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 (2014).
Pelly, V. S. et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 9, 1407–1417 (2016).
Kim, L. K. et al. AMCase is a crucial regulator of type 2 immune responses to inhaled house dust mites. Proc. Natl Acad. Sci. USA 112, E2891–E2899 (2015).
Wiesner, D. L. et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 11, e1004701 (2015).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Van Dyken, S. J. et al. A tissue checkpoint regulates type 2 immunity. Nat. Immunol. 17, 1381–1387 (2016).
Endo, Y. et al. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity 42, 294–308 (2015).
Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 75, 14–24 (2015).
Maier, E., Duschl, A. & Horejs-Hoeck, J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur. J. Immunol. 42, 2827–2833 (2012).
Yagi, R., Zhu, J. & Paul, W. E. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 23, 415–420 (2011).
Zhu, J. Transcriptional regulation of Th2 cell differentiation. Immunol. Cell Biol. 88, 244–249 (2010).
Wei, G. et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 35, 299–311 (2011). This paper describes genome-wide chromatin immunoprecipitation followed by sequencing (ChIP–seq) analysis of GATA3 binding sites and reveals that there are shared and cell-specific patterns of GATA3 function during T cell development and effector function.
Hosokawa, H. et al. Methylation of Gata3 protein at Arg-261 regulates transactivation of the Il5 gene in T helper 2 cells. J. Biol. Chem. 290, 13095–13103 (2015).
Hosokawa, H. et al. Akt1-mediated Gata3 phosphorylation controls the repression of IFNγ in memory-type Th2 cells. Nat. Commun. 7, 11289 (2016).
Hammad, H. & Lambrecht, B. N. Barrier epithelial cells and the control of type 2 immunity. Immunity 43, 29–40 (2015).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. Cell 169, 570–586 (2017).
Buck, M. D., O'Sullivan, D. & Pearce, E. L. T cell metabolism drives immunity. J. Exp. Med. 212, 1345–1360 (2015).
Linke, M., Fritsch, S. D., Sukhbaatar, N., Hengstschläger, M. & Weichhart, T. mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett. http://dx.doi.org/10.1002/1873-3468.12711 (2017).
Delgoffe, G. M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Lee, K. et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32, 743–753 (2010).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 (2011).
Yang, K. et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity 39, 1043–1056 (2013). References 72–75 highlight the importance of cellular metabolism in the differentiation of T H 1 and T H 2 cells.
Huber, M. & Lohoff, M. IRF4 at the crossroads of effector T-cell fate decision. Eur. J. Immunol. 44, 1886–1895 (2014).
Bao, K. et al. BATF modulates the Th2 locus control region and regulates CD4+ T cell fate during antihelminth immunity. J. Immunol. 197, 4371–4381 (2016).
Kuwahara, M. et al. Bach2-Batf interactions control Th2-type immune response by regulating the IL-4 amplification loop. Nat. Commun. 7, 12596 (2016).
Iwata, A. et al. Quality of TCR signaling determined by differential affinities of enhancers for the composite BATF-IRF4 transcription factor complex. Nat. Immunol. 18, 563–572 (2017). This study provides insight into how TCR-induced variations in the abundance of BATF–IRF4 complexes translate into different patterns of gene expression.
Sahoo, A. et al. Batf is important for IL-4 expression in T follicular helper cells. Nat. Commun. 6, 7997 (2015).
Li, P. et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature 490, 543–546 (2012).
Ando, R. et al. The transcription factor Bach2 is phosphorylated at multiple sites in murine B cells but a single site prevents its nuclear localization. J. Biol. Chem. 291, 1826–1840 M115.661702 (2016).
Bruchard, M. et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat. Immunol. 16, 859–870 (2015).
Nakayama, T. et al. Th2 cells in health and disease. Annu. Rev. Immunol. 35, 53–84 (2017).
Nurieva, R. I. et al. A costimulation-initiated signaling pathway regulates NFATc1 transcription in T lymphocytes. J. Immunol. 179, 1096–1103 (2007).
van Panhuys, N., Klauschen, F. & Germain, R. N. T-Cell-receptor-dependent signal intensity dominantly controls CD4+ T cell polarization in vivo. Immunity 41, 63–74 (2014).
Man, K. et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 14, 1155–1165 (2013).
Yang, C. W. et al. Regulation of T cell receptor signaling by DENND1B in TH2 cells and allergic disease. Cell 164, 141–155 (2016).
Sleiman, P. M. et al. Variants of DENND1B associated with asthma in children. N. Engl. J. Med. 362, 36–44 (2010). Reference 88 shows that DENND1B, a guanine nucleotide exchange factor, is required for the internalization of the TCR specifically in T H 2 cells. Both references 88 and 89 identified polymorphisms in DENND1B that are associated with allergic disease.
Pua, H. H. et al. MicroRNAs 24 and 27 suppress allergic inflammation and target a network of regulators of T helper 2 cell-associated cytokine production. Immunity 44, 821–832 (2016).
Chong, M. M., Rasmussen, J. P., Rudensky, A. Y. & Littman, D. R. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J. Exp. Med. 205, 2005–2017 (2008).
Muljo, S. A. et al. Aberrant T cell differentiation in the absence of Dicer. J. Exp. Med. 202, 261–269 (2005).
Okoye, I. S. et al. Transcriptomics identified a critical role for Th2 cell-intrinsic miR-155 in mediating allergy and antihelminth immunity. Proc. Natl Acad. Sci. USA 111, E3081–E3090 (2014).
Cho, S. et al. miR-23 approximately 27 approximately 24 clusters control effector T cell differentiation and function. J. Exp. Med. 213, 235–249 (2016).
Simpson, L. J. et al. A microRNA upregulated in asthma airway T cells promotes TH2 cytokine production. Nat. Immunol. 15, 1162–1170 (2014). References 93–95 demonstrate that miRNAs markedly influence T H 2 cell cytokine responses in disease.
Malmhall, C. et al. MicroRNA-155 is essential for TH2-mediated allergen-induced eosinophilic inflammation in the lung. J. Allergy Clin. Immunol. 133, 1429–1438.e7 (2014).
Bala, S. et al. Biodistribution and function of extracellular miRNA-155 in mice. Sci. Rep. 5, 10721 (2015).
Sastre, B., Canas, J. A., Rodrigo-Munoz, J. M. & Del Pozo, V. Novel modulators of asthma and allergy: exosomes and microRNAs. Front. Immunol. 8, 826 (2017).
Buck, A. H. et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 5, 5488 (2014).
Entwistle, L. J. & Wilson, M. S. MicroRNA-mediated regulation of immune responses to intestinal helminth infections. Parasite Immunol. 39, e12406 (2017).
Tumes, D. J. et al. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4+ T helper type 1 and type 2 cells. Immunity 39, 819–832 (2013).
Allan, R. S. et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature 487, 249–253 (2012).
Hawkins, R. D. et al. Global chromatin state analysis reveals lineage-specific enhancers during the initiation of human T helper 1 and T helper 2 cell polarization. Immunity 38, 1271–1284 (2013).
Seumois, G. et al. Epigenomic analysis of primary human T cells reveals enhancers associated with TH2 memory cell differentiation and asthma susceptibility. Nat. Immunol. 15, 777–788 (2014). References 101–104 enhance our understanding of how epigenetic factors modulate T H cell differentiation in health and disease.
Peine, M. et al. Stable T-bet+GATA-3+ Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS Biol. 11, e1001633 (2013).
Wang, Y. H. et al. A novel subset of CD4+ TH2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J. Exp. Med. 207, 2479–2491 (2010).
Oestreich, K. J. & Weinmann, A. S. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nat. Rev. Immunol. 12, 799–804 (2012).
Ballesteros-Tato, A. et al. T follicular helper cell plasticity shapes pathogenic T helper 2 cell-mediated immunity to inhaled house dust mite. Immunity 44, 259–273 (2016).
Luthje, K. et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 13, 491–498 (2012).
Stubbington, M. J. et al. T cell fate and clonality inference from single-cell transcriptomes. Nat. Methods 13, 329–332 (2016). This paper describes methods to identify clonally related T cells within single-cell RNA sequencing data.
Mahata, B. et al. Single-cell RNA sequencing reveals T helper cells synthesizing steroids de novo to contribute to immune homeostasis. Cell Rep. 7, 1130–1142 (2014).
Cheng, Y., Wong, M. T., van der Maaten, L. & Newell, E. W. Categorical analysis of human T cell heterogeneity with one-dimensional soli-expression by nonlinear stochastic embedding. J. Immunol. 196, 924–932 (2016).
Koch, M. A. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 (2009).
Levine, A. G. et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature 546, 421–425 (2017).
Chaudhry, A. et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326, 986–991 (2009).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Zheng, Y. et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 458, 351–356 (2009).
Wohlfert, E. A. et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J. Clin. Invest. 121, 4503–4515 (2011).
Cretney, E. et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–311 (2011).
Wang, Y., Su, M. A. & Wan, Y. Y. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 35, 337–348 (2011).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Wu, C. et al. The transcription factor musculin promotes the unidirectional development of peripheral Treg cells by suppressing the TH2 transcriptional program. Nat. Immunol. 18, 344–353 (2017).
Pelly, V. S. et al. Interleukin 4 promotes the development of ex-Foxp3 Th2 cells during immunity to intestinal helminths. J. Exp. Med. 214, 1809–1826 (2017).
Flood-Page, P. T., Menzies-Gow, A. N., Kay, A. B. & Robinson, D. S. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med. 167, 199–204 (2003).
Leckie, M. J. et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356, 2144–2148 (2000).
Ortega, H. G. et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 371, 1198–1207 (2014).
Busse, W. W., Ring, J., Huss-Marp, J. & Kahn, J. E. A review of treatment with mepolizumab, an anti-IL-5 mAb, in hypereosinophilic syndromes and asthma. J. Allergy Clin. Immunol. 125, 803–813 (2010).
Pavord, I. D. et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 380, 651–659 (2012).
Rothenberg, M. E. et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N. Engl. J. Med. 358, 1215–1228 (2008). References 124–129 describe how the passage of anti-IL-5 therapeutic antibodies into the clinic was ultimately successful.
Hanania, N. A. et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 4, 781–796 (2016).
Brightling, C. E. et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 3, 692–701 (2015).
Wenzel, S. et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 388, 31–44 (2016).
Simpson, E. L. et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 375, 2335–2348 (2016). References 132 and 133 illustrate how the combined blockade of the IL-4–IL-13 signalling pathway is beneficial in asthma and atopic dermatitis.
McKenzie, A. N. Regulation of Th2 immunity by interleukin-4 and interleukin-13. Pharmacol. Ther. 88, 143–151 (2000).
Lloyd, C. M. & Saglani, S. Epithelial cytokines and pulmonary allergic inflammation. Curr. Opin. Immunol. 34, 52–58 (2015).
Gauvreau, G. M. et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 370, 2102–2110 (2014).
Corren, J. et al. Tezepelumab in adults with uncontrolled asthma. N. Engl. J. Med. 377, 936–946 (2017). This is the first report to show that targeting an epithelial cell-derived cytokine (namely, TSLP) can reduce asthma exacerbations.
Scott, I. C., Houslay, K. F. & Cohen, E. S. Prospects to translate the biology of IL-33 and ST2 during organ transplantation into therapeutics to treat graft-versus-host disease. Ann. Transl Med. 4, 500 (2016).
Beale, J. et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci. Transl Med. 6, 256ra134 (2014).
Lam, E. P. et al. IL-25/IL-33-responsive TH2 cells characterize nasal polyps with a default TH17 signature in nasal mucosa. J. Allergy Clin. Immunol. 137, 1514–1524 (2016).
Shin, H. W. et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 135, 1476–1485.e7 (2015).
Lee, M., Kim, D. W. & Shin, H. W. Targeting IL-25 as a novel therapy in chronic rhinosinusitis with nasal polyps. Curr. Opin. Allergy Clin. Immunol. 17, 17–22 (2017).
Ballantyne, S. J. et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 120, 1324–1331 (2007).
Stinson, S. E., Amrani, Y. & Brightling, C. E. D prostanoid receptor 2 (chemoattractant receptor-homologous molecule expressed on TH2 cells) protein expression in asthmatic patients and its effects on bronchial epithelial cells. J. Allergy Clin. Immunol. 135, 395–406 (2015).
Barnes, N. et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin. Exp. Allergy 42, 38–48 (2012).
Hall, I. P. et al. Efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as sole controller and in the presence of inhaled corticosteroid treatment. Pulm. Pharmacol. Ther. 32, 37–44 (2015).
Kuna, P., Bjermer, L. & Tornling, G. Two phase II randomized trials on the CRTh2 antagonist AZD1981 in adults with asthma. Drug Des. Devel. Ther. 10, 2759–2770 (2016).
Gonem, S. et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir. Med. 4, 699–707 (2016).
Huang, T. et al. Depletion of major pathogenic cells in asthma by targeting CRTh2. JCI Insight 1, e86689 (2016).
Chiba, Y., Todoroki, M., Nishida, Y., Tanabe, M. & Misawa, M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am. J. Respir. Cell Mol. Biol. 41, 516–524 (2009).
Nagashima, S. et al. Novel 7H-pyrrolo[2,3-d]pyrimidine derivatives as potent and orally active STAT6 inhibitors. Bioorg. Med. Chem. 17, 6926–6936 (2009).
Ohga, K. et al. YM-341619 suppresses the differentiation of spleen T cells into Th2 cells in vitro, eosinophilia, and airway hyperresponsiveness in rat allergic models. Eur. J. Pharmacol. 590, 409–416 (2008).
Sel, S. et al. Effective prevention and therapy of experimental allergic asthma using a GATA-3-specific DNAzyme. J. Allergy Clin. Immunol. 121, 910–916.e5 (2008).
Garn, H. & Renz, H. GATA-3-specific DNAzyme — a novel approach for stratified asthma therapy. Eur. J. Immunol. 47, 22–30 (2017).
Krug, N. et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N. Engl. J. Med. 372, 1987–1995 (2015). This study describes a novel approach to asthma therapy that targets the transcription factor GATA3.
Drake, M. G., Kaufman, E. H., Fryer, A. D. & Jacoby, D. B. The therapeutic potential of Toll-like receptor 7 stimulation in asthma. Inflamm. Allergy Drug Targets 11, 484–491 (2012).
Dong, Z. et al. Holding the inflammatory system in check: TLRs and their targeted therapy in asthma. Mediators Inflamm. 2016, 2180417 (2016).
Xirakia, C. et al. Toll-like receptor 7-triggered immune response in the lung mediates acute and long-lasting suppression of experimental asthma. Am. J. Respir. Crit. Care Med. 181, 1207–1216 (2010).
Pockros, P. J. et al. Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J. Hepatol. 47, 174–182 (2007).
Beeh, K. M. et al. The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J. Allergy Clin. Immunol. 131, 866–874 (2013).
Casale, T. B. et al. CYT003, a TLR9 agonist, in persistent allergic asthma — a randomized placebo-controlled Phase 2b study. Allergy 70, 1160–1168 (2015).
Creticos, P. S. et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N. Engl. J. Med. 355, 1445–1455 (2006).
Laffont, S. et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J. Exp. Med. 214, 1581 (2017).
Fort, M. M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Ikeda, K. et al. Mast cells produce interleukin-25 upon FcɛRI-mediated activation. Blood 101, 3594–3596 (2003).
Kang, C. M. et al. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am. J. Respir. Cell Mol. Biol. 33, 290–296 (2005).
Wang, Y. H. et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 204, 1837–1847 (2007).
von Moltke, J., Ji, M., Liang, H. E. & Locksley, R. M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016).
Carriere, V. et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl Acad. Sci. USA 104, 282–287 (2007).
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Xu, D. et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 187, 787–794 (1998).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Murakami-Satsutani, N. et al. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol. Int. 63, 443–455 (2014).
Allakhverdi, Z., Smith, D. E., Comeau, M. R. & Delespesse, G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J. Immunol. 179, 2051–2054 (2007).
Ho, L. H. et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcɛRI signals. J. Leukoc. Biol. 82, 1481–1490 (2007).
Iikura, M. et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Invest. 87, 971–978 (2007).
Ziegler, S. F. et al. The biology of thymic stromal lymphopoietin (TSLP). Adv. Pharmacol. 66, 129–155 (2013).
Watanabe, N. et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat. Immunol. 5, 426–434 (2004).
Wang, Y. H. et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity 24, 827–838 (2006).
Zhou, B. et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6, 1047–1053 (2005).
Hondowicz, B. D. et al. Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity 44, 155–166 (2016).
Islam, S. A. et al. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ TH2 cells. Nat. Immunol. 12, 167–177 (2011).
Endo, Y., Hirahara, K., Yagi, R., Tumes, D. J. & Nakayama, T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 35, 69–78 (2014).
Guo, L. et al. Innate immunological function of TH2 cells in vivo. Nat. Immunol. 16, 1051–1059 (2015).
Acknowledgements
The authors apologize to their colleagues whose excellent work they were unable to include due to space limitations. The authors are grateful to P. Fallon and J. Barlow for their thoughtful and insightful suggestions. J.A.W. and A.N.J.M. are funded by the Wellcome Trust (100963/Z/13/Z) and the Medical Research Council (U105178805).
Author information
Authors and Affiliations
Contributions
A.N.J.M. and J.A.W. researched data for the article, contributed to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.N.J.M. has received grant funding from GlaxoSmithKline and MedImmune.
Glossary
- Innate lymphoid cell
-
(ILC). A cytokine-producing lymphocyte that, unlike T and B cells, does not express an antigen-specific receptor.
- Trogocytosis
-
The process through which cells extract membrane fragments from neighbouring cells and exhibit them on their own surface membrane.
- TH2 cytokine locus
-
The gene locus that harbours the genes encoding the cytokines IL-4, IL-5 and IL-13.
- Importin
-
A protein that binds to specific nuclear localization sequences to facilitate the transport of other proteins into the nucleus.
- In vitro-induced Treg (iTreg) cells
-
Regulatory T cells that can be induced in vitro from naive CD4+ T cells in the presence of transforming growth factor-β (TGFβ).
- Endotypes
-
Distinct subtypes of a disease.
Rights and permissions
About this article
Cite this article
Walker, J., McKenzie, A. TH2 cell development and function. Nat Rev Immunol 18, 121–133 (2018). https://doi.org/10.1038/nri.2017.118
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2017.118
This article is cited by
-
Exosome-mediated regulation of inflammatory pathway during respiratory viral disease
Virology Journal (2024)
-
Integrative multi-omics analysis identifies genetically supported druggable targets and immune cell specificity for myasthenia gravis
Journal of Translational Medicine (2024)
-
TCF1–LEF1 co-expression identifies a multipotent progenitor cell (TH2-MPP) across human allergic diseases
Nature Immunology (2024)
-
Mesenchymal stem cells can prevent or promote the progression of colon cancer based on their timing of administration
Journal of Translational Medicine (2023)
-
Exploration and validation of a combined Hypoxia and m6A/m5C/m1A regulated gene signature for prognosis prediction of liver cancer
BMC Genomics (2023)