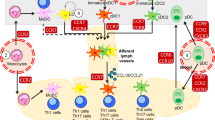
Key Points
-
During embryonic development and postnatally, dendritic cell (DC) progenitors migrate into non-lymphoid organs and differentiate into immature DCs.
-
Immature DCs form a dense network of sentinel cells at all outer and inner surfaces of the body, as well as in most organs.
-
Immature DCs sample and process both self and foreign antigens. They subsequently undergo an activation process that is triggered by either an 'intrinsic programme' or in response to the recognition of molecular patterns associated with pathogens and the microbiota.
-
As part of the activation programme, DCs upregulate CC-chemokine receptor 7 (CCR7) and increase their motility. The CCR7 ligand CC-chemokine ligand 21 (CCL21) is expressed on terminal lymphatics and CCR7–CCL21 interactions enable DCs to enter the lymphatic vasculature and eventually the draining lymph node, where they migrate into the T cell-rich paracortex.
-
Within lymph nodes and other lymphoid organs, DCs present antigen to T cells, leading either to the induction of immunological tolerance or to the expansion of protective pro-inflammatory effector and memory T cell populations. In some cases, DC-mediated presentation of self or harmless foreign antigens leads to the formation of effector T cell populations; as such, DCs can contribute to the development of autoimmune or allergic diseases.
-
Effector T cells that develop during protective immune responses home to the tissue site of infection and inflammation and frequently contribute to the recruitment of further DC progenitors. Following their differentiation, such progenitors can present antigen to T cells, either locally or — after mobilization — in draining lymph nodes, thus amplifying protective as well as detrimental immune responses.
Abstract
Dendritic cells (DCs) are potent and versatile antigen-presenting cells, and their ability to migrate is key for the initiation of protective pro-inflammatory as well as tolerogenic immune responses. Recent comprehensive studies have highlighted the importance of DC migration in the maintenance of immune surveillance and tissue homeostasis, and also in the pathogenesis of a range of diseases. In this Review, we summarize the anatomical, cellular and molecular factors that regulate the migration of different DC subsets in health and disease. In particular, we focus on new insights concerning the role of migratory DCs in the pathogenesis of diseases of the skin, intestine, lung, and brain, as well as in autoimmunity and atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Steinman, R. M. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30, 1–22 (2012).
Becher, B. et al. High-dimensional analysis of the murine myeloid cell system. Nat. Immunol. 15, 1181–1189 (2014).
Murphy, T. L. et al. Transcriptional control of dendritic cell development. Annu. Rev. Immunol. 34, 93–119 (2016).
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Guilliams, M. et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14, 571–578 (2014).
Guilliams, M. & van de Laar, L. A hitchhiker's guide to myeloid cell subsets: practical implementation of a novel mononuclear phagocyte classification system. Front. Immunol. 6, 406 (2015).
Reynolds, G. & Haniffa, M. Human and mouse mononuclear phagocyte networks: a tale of two species? Front. Immunol. 6, 330 (2015).
Merad, M., Sathe, P., Helft, J., Miller, J. & Mortha, A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31, 563–604 (2013).
Mildner, A. & Jung, S. Development and function of dendritic cell subsets. Immunity 40, 642–656 (2014).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Tal, O. et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J. Exp. Med. 208, 2141–2153 (2011).
Weber, M. et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339, 328–332 (2013). This study identified endogenous gradients of immobilized CCL21 within the skin that guide migrating DCs towards and into initial lymphatics by means of CCR7-dependent haptotactic directional cues.
Pflicke, H. & Sixt, M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 206, 2925–2935 (2009).
Rescigno, M., Martino, M., Sutherland, C. L., Gold, M. R. & Ricciardi-Castagnoli, P. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 188, 2175–2180 (1998).
Krappmann, D. et al. The IκB kinase complex and NF-κB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol. Cell. Biol. 24, 6488–6500 (2004).
Baratin, M. et al. Homeostatic NF-κB signaling in steady-state migratory dendritic cells regulates immune homeostasis and tolerance. Immunity 42, 627–639 (2015).
Braun, A. et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 12, 879–887 (2011).
Ulvmar, M. H. et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 15, 623–630 (2014). This manuscript shows the active formation of a chemokine gradient shaped by an atypical chemokine receptor.
Lämmermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Wendland, M. et al. Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity 35, 945–957 (2011).
Qu, C. et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J. Exp. Med. 200, 1231–1241 (2004).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Liu, K. et al. In vivo analysis of dendritic cell development and homeostasis. Science 324, 392–397 (2009).
Seth, S. et al. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J. Immunol. 186, 3364–3372 (2011).
Gatto, D. et al. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat. Immunol. 14, 446–453 (2013).
Yi, T. & Cyster, J. G. EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. eLife 2, e00757 (2013).
León, B. et al. Regulation of TH2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat. Immunol. 13, 681–690 (2012).
Woodruff, M. C. et al. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J. Exp. Med. 211, 1611–1621 (2014).
Gonzalez, S. F. et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat. Immunol. 11, 427–434 (2010).
Tan, S.-Y., Roediger, B. & Weninger, W. The role of chemokines in cutaneous immunosurveillance. Immunol. Cell Biol. 93, 337–346 (2015).
Malissen, B., Tamoutounour, S. & Henri, S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 14, 417–428 (2014).
Wang, Y. et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13, 753–760 (2012).
Kaplan, D. H., Jenison, M. C., Saeland, S., Shlomchik, W. D. & Shlomchik, M. J. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23, 611–620 (2005).
Ginhoux, F. et al. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7, 265–273 (2006).
Nagao, K. et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 13, 744–752 (2012).
Bobr, A. et al. Autocrine/paracrine TGF-β1 inhibits Langerhans cell migration. Proc. Natl Acad. Sci. USA 109, 10492–10497 (2012).
Kissenpfennig, A. et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22, 643–654 (2005).
Shklovskaya, E. et al. Langerhans cells are precommitted to immune tolerance induction. Proc. Natl Acad. Sci. USA 108, 18049–18054 (2011).
Flacher, V. et al. Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol. Med. 6, 1191–1204 (2014).
Gomez de Agüero, M. et al. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8+ T cells and activating Foxp3+ regulatory T cells. J. Clin. Invest. 122, 1700–1711 (2012).
Gaiser, M. R. et al. Cancer-associated epithelial cell adhesion molecule (EpCAM; CD326) enables epidermal Langerhans cell motility and migration in vivo. Proc. Natl Acad. Sci. USA 109, E889–E897 (2012).
Kautz-Neu, K. et al. Langerhans cells are negative regulators of the anti-Leishmania response. J. Exp. Med. 208, 885–891 (2011).
Igyártó, B. Z. et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272 (2011).
Schlitzer, A. et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat. Immunol. 16, 718–728 (2015).
Henri, S. et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 207, 189–206 (2010).
Bedoui, S. et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10, 488–495 (2009).
Murphy, T. L., Tussiwand, R. & Murphy, K. M. Specificity through cooperation: BATF–IRF interactions control immune-regulatory networks. Nat. Rev. Immunol. 13, 499–509 (2013).
Naik, S. et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 (2015).
Kitajima, M. & Ziegler, S. F. Cutting edge: identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity. J. Immunol. 191, 4903–4907 (2013).
Stutte, S. et al. Requirement of CCL17 for CCR7- and CXCR4-dependent migration of cutaneous dendritic cells. Proc. Natl Acad. Sci. USA 107, 8736–8741 (2010).
Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938 (2013).
Mollah, S. A. et al. Flt3L dependence helps define an uncharacterized subset of murine cutaneous dendritic cells. J. Invest. Dermatol. 134, 1265–1275 (2014).
Pascale, F. et al. Plasmacytoid dendritic cells migrate in afferent skin lymph. J. Immunol. 180, 5963–5972 (2008).
Sisirak, V. et al. CCR6/CCR10-mediated plasmacytoid dendritic cell recruitment to inflamed epithelia after instruction in lymphoid tissues. Blood 118, 5130–5140 (2011).
Davalos-Misslitz, A. C. M. et al. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur. J. Immunol. 37, 613–622 (2007).
Bajaña, S., Roach, K., Turner, S., Paul, J. & Kovats, S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J. Immunol. 189, 3368–3377 (2012).
Yabe, R. et al. CCR8 regulates contact hypersensitivity by restricting cutaneous dendritic cell migration to the draining lymph nodes. Int. Immunol. 27, 169–181 (2015).
Sawada, Y. et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 212, 1921–1930 (2015). Here the authors demonstrate that resolvin E1, a lipid mediator derived from ω3 polyunsaturated fatty acids, impairs DC motility in the skin.
Tomura, M. et al. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci. Rep. 4, 6030 (2014).
Girard, J.-P., Moussion, C. & Förster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 12, 762–773 (2012).
Cook, D. N. et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12, 495–503 (2000).
Shreedhar, V. K., Kelsall, B. L. & Neutra, M. R. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T− and B-cell areas of Peyer's patches. Infect. Immun. 71, 504–509 (2003).
Salazar-Gonzalez, R. M. et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity 24, 623–632 (2006).
Lopez-Guerrero, D. V. et al. Rotavirus infection activates dendritic cells from Peyer's patches in adult mice. J. Virol. 84, 1856–1866 (2010).
Cerovic, V. et al. Intestinal CD103− dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 6, 104–113 (2013).
Pabst, O. et al. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J. Immunol. 177, 6824–6832 (2006).
Schulz, O. et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 206, 3101–3114 (2009).
Yrlid, U. et al. Regulation of intestinal dendritic cell migration and activation by plasmacytoid dendritic cells, TNF-α and type 1 IFNs after feeding a TLR7/8 ligand. J. Immunol. 176, 5205–5212 (2006).
Persson, E. K. et al. IRF4 transcription-factor-dependent CD103+CD11b+ dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38, 958–969 (2013).
Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Sun, C.-M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Johansson-Lindbom, B. et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 (2005).
Jaensson, E. et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205, 2139–2149 (2008).
Scott, C. L. et al. CCR2+CD103− intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol. 8, 327–339 (2015).
Diehl, G. E. et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX3CR1hi cells. Nature 494, 116–120 (2013).
Worbs, T. et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203, 519–527 (2006).
Farache, J. et al. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38, 581–595 (2013).
Niess, J. H. & Reinecker, H.-C. Lamina propria dendritic cells in the physiology and pathology of the gastrointestinal tract. Curr. Opin. Gastroenterol. 21, 687–691 (2005).
McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012).
Arques, J. L. et al. Salmonella induces flagellin- and MyD88-dependent migration of bacteria-capturing dendritic cells into the gut lumen. Gastroenterology 137, 579–587. e2 (2009).
Yrlid, U. et al. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J. Immunol. 177, 6115–6121 (2006).
Wendland, M. et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc. Natl Acad. Sci. USA 104, 6347–6352 (2007).
Goubier, A. et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29, 464–475 (2008).
Mizuno, S. et al. CCR9+ plasmacytoid dendritic cells in the small intestine suppress development of intestinal inflammation in mice. Immunol. Lett. 146, 64–69 (2012).
Baumgart, D. C. et al. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn's disease and ulcerative colitis. Clin. Exp. Immunol. 166, 46–54 (2011).
Kwa, S. et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood 118, 2763–2773 (2011).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013).
Tamoutounour, S. et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 42, 3150–3166 (2012).
Dunay, I. R. et al. Gr1+ inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29, 306–317 (2008).
Schreiber, H. A. et al. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J. Exp. Med. 210, 2025–2039 (2013).
Rivollier, A., He, J., Kole, A., Valatas, V. & Kelsall, B. L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209, 139–155 (2012).
Zigmond, E. et al. Ly6Chi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090 (2012).
Siddiqui, K. R. R., Laffont, S. & Powrie, F. E-Cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity 32, 557–567 (2010).
Langlet, C. et al. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J. Immunol. 188, 1751–1760 (2012).
Esterházy, D. et al. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral Treg cells and tolerance. Nat. Immunol. 17, 545–555 (2016).
Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6, 507–514 (2005).
Jaensson-Gyllenbäck, E. et al. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 4, 438–447 (2011).
McDonald, K. G. et al. Epithelial expression of the cytosolic retinoid chaperone cellular retinol binding protein II is essential for in vivo imprinting of local gut dendritic cells by lumenal retinoids. Am. J. Pathol. 180, 984–997 (2012).
Laffont, S., Siddiqui, K. R. R. & Powrie, F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur. J. Immunol. 40, 1877–1883 (2010).
Zhang, Z. et al. Peripheral lymphoid volume expansion and maintenance are controlled by gut microbiota via RALDH+ dendritic cells. Immunity 44, 330–342 (2016). This study shows that neonatal DCs in the gut respond to microbial colonization and migrate to cutaneous lymph nodes, where they instruct HEV maturation for the initiation of L-selectin-based homing of lymphocytes and lymph node cellularity increase.
Macpherson, A. J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40, 594–607 (2014).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Fonseca, D. M. da et al. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell 163, 354–366 (2015).
Voedisch, S. et al. Mesenteric lymph nodes confine dendritic cell-mediated dissemination of Salmonella enterica serovar Typhimurium and limit systemic disease in mice. Infect. Immun. 77, 3170–3180 (2009).
Uematsu, S. et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 7, 868–874 (2006).
del Rio, M.-L., Rodriguez-Barbosa, J.-I., Kremmer, E. & Förster, R. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 178, 6861–6866 (2007).
Hintzen, G. et al. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J. Immunol. 177, 7346–7354 (2006).
Kandasamy, M. et al. Complement mediated signaling on pulmonary CD103+ dendritic cells is critical for their migratory function in response to influenza infection. PLoS Pathog. 9, e1003115 (2013).
Jakubzick, C., Tacke, F., Llodra, J., van Rooijen, N. & Randolph, G. J. Modulation of dendritic cell trafficking to and from the airways. J. Immunol. 176, 3578–3584 (2006).
Otero, K. et al. Nonredundant role of CCRL2 in lung dendritic cell trafficking. Blood 116, 2942–2949 (2010).
Idzko, M. et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J. Clin. Invest. 116, 2935–2944 (2006).
Hammad, H. et al. Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J. Immunol. 171, 3936–3940 (2003).
Zhao, J. J., Zhao, J. J., Legge, K. & Perlman, S. Age-related increases in PGD2 expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Invest. 121, 4921–4930 (2011).
Le Nouën, C. et al. Low CCR7-mediated migration of human monocyte derived dendritic cells in response to human respiratory syncytial virus and human metapneumovirus. PLoS Pathog. 7, e1002105 (2011).
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA 108, 5354–5359 (2011).
Pang, I. K., Ichinohe, T. & Iwasaki, A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8+ T cell responses to influenza A virus. Nat. Immunol. 14, 246–253 (2013).
Thornton, E. E. et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J. Exp. Med. 209, 1183–1199 (2012).
Hashimoto, M. et al. TGF-β-dependent dendritic cell chemokinesis in murine models of airway disease. J. Immunol. 195, 1182–1190 (2015).
Kitamura, H. et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Invest. 121, 2863–2875 (2011).
Plantinga, M. et al. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38, 322–335 (2013).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Willart, M. A. et al. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J. Exp. Med. 209, 1505–1517 (2012).
Upham, J. W. et al. Plasmacytoid dendritic cells during infancy are inversely associated with childhood respiratory tract infections and wheezing. J. Allergy Clin. Immunol. 124, 707–713. e2 (2009).
de Heer, H. J. et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200, 89–98 (2004).
Kool, M. et al. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J. Immunol. 183, 1074–1082 (2009).
Lombardi, V., Speak, A. O., Kerzerho, J., Szely, N. & Akbari, O. CD8α+β− and CD8α+β+ plasmacytoid dendritic cells induce Foxp3+ regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol. 5, 432–443 (2012).
Khare, A. et al. Cutting edge: inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. J. Immunol. 191, 25–29 (2013).
Vassallo, R. et al. Cigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium study. Respir. Res. 11, 45 (2010).
Demedts, I. K. et al. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 175, 998–1005 (2007).
Arellano-Orden, E. et al. Cigarette smoke decreases the maturation of lung myeloid dendritic cells. PLoS ONE 11, e0152737 (2016).
GeurtsvanKessel, C. H. et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 205, 1621–1634 (2008).
Legge, K. L. & Braciale, T. J. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18, 265–277 (2003).
Helft, J. et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J. Clin. Invest. 122, 4037–4047 (2012).
Ho, A. W. S. et al. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J. Immunol. 187, 6011–6021 (2011).
Guilliams, M., Lambrecht, B. N. & Hammad, H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 6, 464–473 (2013).
Ballesteros-Tato, A., León, B., Lund, F. E. & Randall, T. D. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8+ T cell responses to influenza. Nat. Immunol. 11, 216–224 (2010).
Lukens, M. V., Kruijsen, D., Coenjaerts, F. E. J., Kimpen, J. L. L. & van Bleek, G. M. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J. Virol. 83, 7235–7243 (2009).
Lin, K. L., Suzuki, Y., Nakano, H., Ramsburg, E. & Gunn, M. D. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J. Immunol. 180, 2562–2572 (2008).
Nakano, H. et al. Migratory properties of pulmonary dendritic cells are determined by their developmental lineage. Mucosal Immunol. 6, 678–691 (2013).
Iijima, N., Mattei, L. M. & Iwasaki, A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc. Natl Acad. Sci. USA 108, 284–289 (2011).
Cao, W. et al. Rapid differentiation of monocytes into type I IFN-producing myeloid dendritic cells as an antiviral strategy against influenza virus infection. J. Immunol. 189, 2257–2265 (2012).
Khader, S. A. et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J. Exp. Med. 203, 1805–1815 (2006).
Shafiani, S., Tucker-Heard, G., Kariyone, A., Takatsu, K. & Urdahl, K. B. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 207, 1409–1420 (2010).
Curtis, J. et al. Susceptibility to tuberculosis is associated with variants in the ASAP1 gene encoding a regulator of dendritic cell migration. Nat. Genet. 47, 523–527 (2015).
Cleret, A. et al. Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J. Immunol. 178, 7994–8001 (2007).
Medawar, P. B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 29, 58–69 (1948).
Bentivoglio, M. & Kristensson, K. Tryps and trips: cell trafficking across the 100-year-old blood-brain barrier. Trends Neurosci. 37, 325–333 (2014).
Ransohoff, R. M. & Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 12, 623–635 (2012).
Laman, J. D. & Weller, R. O. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J. Neuroimmune Pharmacol. 8, 840–856 (2013).
Louveau, A., Harris, T. H. & Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 36, 569–577 (2015).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). Characterizing functional lymphatic vessels within the meninges, these two studies have shed new light on controversial questions of lymphatic drainage and, even more, cell-bound antigen-transport from the CNS, thus revisiting classic dogmas of CNS immune privilege.
Quintana, E. et al. DNGR-1+ dendritic cells are located in meningeal membrane and choroid plexus of the noninjured brain. Glia 63, 2231–2248 (2015).
Hatterer, E., Touret, M., Belin, M.-F., Honnorat, J. & Nataf, S. Cerebrospinal fluid dendritic cells infiltrate the brain parenchyma and target the cervical lymph nodes under neuroinflammatory conditions. PLoS ONE 3, e3321 (2008).
Jain, P., Coisne, C., Enzmann, G., Rottapel, R. & Engelhardt, B. α4β1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J. Immunol. 184, 7196–7206 (2010).
Paterka, M. et al. Gatekeeper role of brain antigen-presenting CD11c+ cells in neuroinflammation. EMBO J. 35, 89–101 (2016).
Bartholomäus, I. et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98 (2009).
Clarkson, B. D. et al. CCR2-dependent dendritic cell accumulation in the central nervous system during early effector experimental autoimmune encephalomyelitis is essential for effector T cell restimulation in situ and disease progression. J. Immunol. 194, 531–541 (2015).
Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. V. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014).
Duraes, F. V. et al. pDC therapy induces recovery from EAE by recruiting endogenous pDC to sites of CNS inflammation. J. Autoimmun. 67, 8–18 (2015).
Karni, A. et al. Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J. Immunol. 177, 4196–4202 (2006).
Thewissen, K. et al. Circulating dendritic cells of multiple sclerosis patients are proinflammatory and their frequency is correlated with MS-associated genetic risk factors. Mult. Scler. 20, 548–557 (2014).
Pashenkov, M. et al. Elevated expression of CCR5 by myeloid (CD11c+) blood dendritic cells in multiple sclerosis and acute optic neuritis. Clin. Exp. Immunol. 127, 519–526 (2002).
Kivisäkk, P. et al. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann. Neurol. 55, 627–638 (2004).
Aung, L. L., Fitzgerald-Bocarsly, P., Dhib-Jalbut, S. & Balashov, K. Plasmacytoid dendritic cells in multiple sclerosis: chemokine and chemokine receptor modulation by interferon-beta. J. Neuroimmunol. 226, 158–164 (2010).
Mohammad, M. G. et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J. Clin. Invest. 124, 1228–1241 (2014). Although its relevance in adult humans remains controversial, this study demonstrated that the RMS is an important migration pathway within the CNS parenchyma of rodents, not only for neurons repopulating the olfactory bulb but for CNS-emigrating DCs as well.
Ganguly, D., Haak, S., Sisirak, V. & Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 13, 566–577 (2013).
Vitali, C. et al. Migratory, and not lymphoid-resident, dendritic cells maintain peripheral self-tolerance and prevent autoimmunity via induction of iTreg cells. Blood 120, 1237–1245 (2012).
Ochando, J. C. et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7, 652–662 (2006).
Bonasio, R. et al. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat. Immunol. 7, 1092–1100 (2006).
Hadeiba, H. et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity 36, 438–450 (2012).
Page, G., Lebecque, S. & Miossec, P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J. Immunol. 168, 5333–5341 (2002).
Tarrant, T. K. et al. Decreased Th17 and antigen-specific humoral responses in CX3CR1-deficient mice in the collagen-induced arthritis model. Arthritis Rheum. 64, 1379–1387 (2012).
Yokoyama, W. et al. Abrogation of CC chemokine receptor 9 ameliorates collagen-induced arthritis of mice. Arthritis Res. Ther. 16, 445 (2014).
Li, X. et al. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J. Cell. Mol. Med. 20, 170–180 (2015).
Ibarra, J. M. et al. CD8α+ dendritic cells improve collagen-induced arthritis in CC chemokine receptor (CCR)-2 deficient mice. Immunobiology 216, 971–978 (2011).
Han, Y. et al. FTY720 abrogates collagen-induced arthritis by hindering dendritic cell migration to local lymph nodes. J. Immunol. 195, 4126–4135 (2015).
Rowland, S. L. et al. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 211, 1977–1991 (2014).
Sisirak, V. et al. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 211, 1969–1976 (2014).
Baccala, R. et al. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc. Natl Acad. Sci. USA 110, 2940–2945 (2013).
Blomberg, S. et al. Presence of cutaneous interferon-a producing cells in patients with systemic lupus erythematosus. Lupus 10, 484–490 (2001).
Khan, S. A. et al. Active systemic lupus erythematosus is associated with decreased blood conventional dendritic cells. Exp. Mol. Pathol. 95, 121–123 (2013).
Guiducci, C. et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 207, 2931–2942 (2010).
Celhar, T. et al. RNA sensing by conventional dendritic cells is central to the development of lupus nephritis. Proc. Natl Acad. Sci. USA 112, E6195–E6204 (2015).
Hänsel, A. et al. Human 6-sulfo LacNAc (slan) dendritic cells have molecular and functional features of an important pro-inflammatory cell type in lupus erythematosus. J. Autoimmun. 40, 1–8 (2013).
Clatworthy, M. R. et al. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nat. Med. 20, 1458–1463 (2014). This paper reveals that immune complexes that are frequently found in autoimmune diseases induce DC mobilization thus potentially contributing to aggravation of disease.
Rodriguez-Pla, A. et al. IFN priming is necessary but not sufficient to turn on a migratory dendritic cell program in lupus monocytes. J. Immunol. 192, 5586–5598 (2014).
Perera, G. K., Di Meglio, P. & Nestle, F. O. Psoriasis. Annu. Rev. Pathol. 7, 385–422 (2012).
Guttman-Yassky, E., Nograles, K. E. & Krueger, J. G. Contrasting pathogenesis of atopic dermatitis and psoriasis — part II: immune cell subsets and therapeutic concepts. J. Allergy Clin. Immunol. 127, 1420–1432 (2011).
Wohn, C. et al. Langerinneg conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl Acad. Sci. USA 110, 10723–10728 (2013).
Tortola, L. et al. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J. Clin. Invest. 122, 3965–3976 (2012).
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202, 135–143 (2005).
Albanesi, C. et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 206, 249–258 (2009).
Skrzeczyn´ska-Moncznik, J. et al. Potential role of chemerin in recruitment of plasmacytoid dendritic cells to diseased skin. Biochem. Biophys. Res. Commun. 380, 323–327 (2009).
Gonzalvo-Feo, S. et al. Endothelial cell-derived chemerin promotes dendritic cell transmigration. J. Immunol. 192, 2366–2373 (2014).
Terhorst, D. et al. Dynamics and transcriptomics of skin dendritic cells and macrophages in an imiquimod-induced, biphasic mouse model of psoriasis. J. Immunol. 195, 4953–4961 (2015).
Bosè, F. et al. Inhibition of CCR7/CCL19 axis in lesional skin is a critical event for clinical remission induced by TNF blockade in patients with psoriasis. Am. J. Pathol. 183, 413–421 (2013).
Kim, T.-G. et al. Dermal clusters of mature dendritic cells and T cells are associated with the CCL20/CCR6 chemokine system in chronic psoriasis. J. Invest. Dermatol. 134, 1462–1465 (2014).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Gerner, M. Y., Torabi-Parizi, P. & Germain, R. N. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 42, 172–185 (2015).
Schumann, K. et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32, 703–713 (2010).
Del Prete, A. et al. Defective dendritic cell migration and activation of adaptive immunity in PI3Kγ-deficient mice. EMBO J. 23, 3505–3515 (2004).
van Rijn, A. et al. Semaphorin 7A promotes chemokine-driven dendritic cell migration. J. Immunol. 196, 459–468 (2016).
Myster, F. et al. Viral semaphorin inhibits dendritic cell phagocytosis and migration but is not essential for gammaherpesvirus-induced lymphoproliferation in malignant catarrhal fever. J. Virol. 89, 3630–3647 (2015).
Lämmermann, T. et al. Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood 113, 5703–5710 (2009).
Harada, Y. et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 119, 4451–4461 (2012).
Krishnaswamy, J. K. et al. Coincidental loss of DOCK8 function in NLRP10-deficient and C3H/HeJ mice results in defective dendritic cell migration. Proc. Natl Acad. Sci. USA 112, 3056–3061 (2015).
Gunawan, M. et al. The methyltransferase Ezh2 controls cell adhesion and migration through direct methylation of the extranuclear regulatory protein talin. Nat. Immunol. 16, 505–516 (2015).
Maddaluno, L. et al. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J. Exp. Med. 206, 623–635 (2009).
Sozzani, S., Vermi, W., Del Prete, A. & Facchetti, F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 31, 270–277 (2010).
Faure-André, G. et al. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science 322, 1705–1710 (2008).
Frittoli, E. et al. The signaling adaptor Eps8 is an essential actin capping protein for dendritic cell migration. Immunity 35, 388–399 (2011).
Xu, Y. et al. Dendritic cell motility and T cell activation requires regulation of Rho-cofilin signaling by the Rho-GTPase activating protein myosin IXb. J. Immunol. 192, 3559–3568 (2014).
Lamsoul, I. et al. ASB2α regulates migration of immature dendritic cells. Blood 122, 533–541 (2013).
Ring, S. et al. Regulatory T cell-derived adenosine induces dendritic cell migration through the Epac–Rap1 pathway. J. Immunol. 194, 3735–3744 (2015).
Adkins, I. et al. Bordetella adenylate cyclase toxin differentially modulates Toll-like receptor-stimulated activation, migration and T cell stimulatory capacity of dendritic cells. PLoS ONE 9, e104064 (2014).
Solanes, P. et al. Space exploration by dendritic cells requires maintenance of myosin II activity by IP3 receptor 1. EMBO J. 34, 798–810 (2015).
Vargas, P. et al. Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat. Cell Biol. 18, 43–53 (2016). This study identifies two main actin pools in DCs: one located in the rear drives forward locomotion, whereas the one in the front limits migration and directs antigen capture.
Thiam, H.-R. et al. Perinuclear Arp2/3-driven actin polymerization enables nuclear deformation to facilitate cell migration through complex environments. Nat. Commun. 7, 10997 (2016).
Gartlan, K. H. et al. Tetraspanin CD37 contributes to the initiation of cellular immunity by promoting dendritic cell migration. Eur. J. Immunol. 43, 1208–1219 (2013).
Jones, E. L. et al. Dendritic cell migration and antigen presentation are coordinated by the opposing functions of the tetraspanins CD82 and CD37. J. Immunol. 196, 978–987 (2016).
Srivatsan, S., Swiecki, M., Otero, K., Cella, M. & Shaw, A. S. CD2-associated protein regulates plasmacytoid dendritic cell migration, but is dispensable for their development and cytokine production. J. Immunol. 191, 5933–5940 (2013).
de Noronha, S. et al. Impaired dendritic-cell homing in vivo in the absence of Wiskott-Aldrich syndrome protein. Blood 105, 1590–1597 (2005).
Prete, F. et al. Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells. J. Exp. Med. 210, 355–374 (2013).
Worth, A. J. J. et al. Disease-associated missense mutations in the EVH1 domain disrupt intrinsic WASp function causing dysregulated actin dynamics and impaired dendritic cell migration. Blood 121, 72–84 (2013).
Cybulsky, M. I., Cheong, C. & Robbins, C. S. Macrophages and dendritic cells: partners in atherogenesis. Circ. Res. 118, 637–652 (2016).
Randolph, G. J. Mechanisms that regulate macrophage burden in atherosclerosis. Circ. Res. 114, 1757–1771 (2014).
Jongstra-Bilen, J. et al. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med. 203, 2073–2083 (2006).
Millonig, G. et al. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler. Thromb. Vasc. Biol. 21, 503–508 (2001).
Choi, J.-H. et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J. Exp. Med. 206, 497–505 (2009).
Paulson, K. E. et al. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res. 106, 383–390 (2010).
Koltsova, E. K. et al. Dynamic T cell–APC interactions sustain chronic inflammation in atherosclerosis. J. Clin. Invest. 122, 3114–3126 (2012).
Weber, C. et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Invest. 121, 2898–2910 (2011).
Hu, D. et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin β receptors. Immunity 42, 1100–1115 (2015).
Choi, J.-H. et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 35, 819–831 (2011).
Legein, B. et al. Ablation of CD8α+ dendritic cell mediated cross-presentation does not impact atherosclerosis in hyperlipidemic mice. Sci. Rep. 5, 15414 (2015).
Zhang, Z. et al. Antigen-loaded dendritic cell migration: MR imaging in a pancreatic carcinoma model. Radiology 274, 192–200 (2015).
Kretzschmar, D. et al. Decrease in circulating dendritic cell precursors in patients with peripheral artery disease. Mediators Inflamm. 2015, 450957 (2015).
Van Vré, E. A. et al. Changes in blood dendritic cell counts in relation to type of coronary artery disease and brachial endothelial cell function. Coron. Artery Dis. 21, 87–96 (2010).
Angeli, V. et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574 (2004).
Weitman, E. S. et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS ONE 8, e70703 (2013).
Nickel, T. et al. oxLDL downregulates the dendritic cell homing factors CCR7 and CCL21. Mediators Inflamm. 2012, 320953 (2012).
Luchtefeld, M. et al. Chemokine receptor 7 knockout attenuates atherosclerotic plaque development. Circulation 122, 1621–1628 (2010).
Feig, J. E. et al. Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS ONE 6, e28534 (2011).
Wan, W., Lionakis, M. S., Liu, Q., Roffê, E. & Murphy, P. M. Genetic deletion of chemokine receptor Ccr7 exacerbates atherogenesis in ApoE-deficient mice. Cardiovasc. Res. 97, 580–588 (2013).
Döring, Y. et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125, 1673–1683 (2012).
Sage, A. P. et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation 130, 1363–1373 (2014).
Daissormont, I. T. et al. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ. Res. 109, 1387–1395 (2011).
Randolph, G. J. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr. Opin. Lipidol. 19, 462–468 (2008).
Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 (2010).
Cerovic, V. et al. Lymph-borne CD8α+ dendritic cells are uniquely able to cross-prime CD8+ T cells with antigen acquired from intestinal epithelial cells. Mucosal Immunol. 8, 38–48 (2015).
Welty, N. E. et al. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J. Exp. Med. 210, 2011–2024 (2013).
Desch, A. N. et al. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J. Exp. Med. 208, 1789–1797 (2011).
Fossum, E. et al. Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8+ T-cell responses against influenza virus. Eur. J. Immunol. 45, 624–635 (2015).
Acknowledgements
The work of R.F. is funded by grants of the European Research Council (ERC advanced grant 322645-LYMPHATICS-HOMING), the Deutsche Forschungsgemeinschaft, DFG (SFB900-B1, SFB738-B5, Fo334/2-2, Fo334/5-1) and the State of Lower Saxony (Niedersachsen Research Network on Neuroinfectiology,N-RENNT; and BIOFABRICATION for NIFE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Mononuclear phagocyte system
-
(MPS). The term coined by Van Furth in 1968 comprises myeloid immune cells other than polymorphonuclear granulocytes and initially included monocytes and macrophages, and, following their discovery, dendritic cells.
- Plasmacytoid DCs
-
(pDCs). Initially described as innate immune cells capable of producing large amounts of type I interferons in response to viral stimuli.
- Haptotaxis
-
A persistent directional migration along gradients of a chemoattractant that are immobilized on cells and/or elements of the extracellular matrix, in contrast to classical chemotaxis, which develops in response to gradients in a soluble phase.
- Subcapsular sinus
-
The entry side of the lymph node that is close to the afferent lymph vessels. The ceiling and the floor of the sinus are lined by lymphatic endothelial cells.
- Lymph node paracortex
-
The area of the lymph node between the subcapsular cortex and the medullary cords. The paracortex is a DC- and T cell-rich area with high endothelial venules, where circulating lymphocytes leave the bloodstream to enter the lymph node.
- Langerhans cells
-
(LCs). These epidermal cells are regarded as a DC–macrophage hybrid, as they exhibit characteristics of both types of phagocytes. They are highly migratory, efficiently reaching skin-draining lymph nodes, but are unique among DC subsets in that they arise from embryonic progenitors and are radio-resistant, long-lived and develop independently of FLT3 ligand.
- Conventional DC1s
-
(cDC1s). Subset of conventional DCs that in the mouse comprises CD8α+ DCs in lymphoid organs, as well as CD103+ DCs in non-lymphoid tissues. Importantly, in both, human and mice, cDC1s express the markers XCR1, CLEC9A, and CADM1, and display a developmental dependency on ID2, IRF8, NFIL3, and members of the AP1 transcription factor family, namely BATF, BATF2 and BATF3.
- Conventional DC2s
-
(cDC2s). Subset of conventional DCs that in both, human and mice, is characterized by the expression of CD11b and SIRPα/CD172a while lacking XCR1 and CLEC9A. Transcription factors required for their development include IRF4, NOTCH2, PU.1, RELB, and RBPJ, with the exact combination being in part tissue-dependent.
- Double negative cDCs
-
(DN cDCs). A subset of conventional DCs in the mouse that does not express CD103 or CD11b.
- Yolk sac-derived erythro-myeloid progenitor
-
Progenitor cells that develop in the yolk sac (a membranous sac attached to the embryo) colonize the nascent fetal liver and give rise in a first wave of haematopoiesis to fetal erythrocytes, macrophages, granulocytes and monocytes.
- Peripheral tolerance
-
Central tolerance mechanisms do not eliminate all self-reactive lymphocytes, in part because food antigens and some self-antigens are not presented in the thymus, the site of T cell development. Therefore, peripheral tolerance mechanisms control lymphocytes that first encounter their cognate self-antigens outside of the thymus. These mechanisms include anergy and deletion of self-reactive T cells as well as suppression of autoreactive cells by regulatory T cells.
- Contact hypersensitivity
-
Experimental animal model for human allergic contact dermatitis. Upon painting a hapten onto the skin, skin DCs migrate to the lymph nodes where they activate hapten-specific T cells. Re-exposure to the same hapten results in the recruitment of specific T cells to the dermis, which triggers the inflammatory process that is responsible for the cutaneous lesion. Contact hypersensitivity and allergic contact dermatitis are examples of type IV hypersensitivity reactions (T cell-mediated allergic reactions).
- Cross-presentation
-
The ability to present extracellular antigens on MHCI molecules to CD8+ T cells. It is required for triggering immune responses against viruses that do not infect antigen-presenting cells.
- Resolvin E1
-
An endogenous anti-inflammatory lipid mediator biosynthesized from the ω-3 polyunsaturated fatty acid eicosapentaenoic acid during the resolution phase of acute inflammation. Also binding to the chemokine-like receptor CMKLR1, it attenuates inflammation in several disease models, including peritonitis, polymicrobial sepsis and allergic airway inflammation.
- Amoeboid migration
-
Mode of rapid motility that is driven by actin-rich pseudopods, hydrostatically generated blebs and a highly contractile uropod. It is characterized by weak or absent adhesion and little or no extracellular matrix proteolysis. DCs, lymphocytes, and cancer cells exhibit amoeboid motility.
- Immune privilege
-
The observation that foreign antigens and allografts introduced into certain sites of the body do not (or do so only very slowly) elicit (cellular) adaptive immune reactions. These sites include the CNS, eyes, testicles, placenta and fetus. However, at least for the CNS, the idea of an 'absolute' immune privilege is no longer valid, as immune surveillance of the CNS does occur within certain limitations.
- Blood–brain barrier
-
Owing to the specialized composition of the so-called neurovascular unit (comprising endothelial cells, pericyte, and astrocyte endfeet) the permeability of blood vessels for plasma components, blood cells and pathogens is much lower in the brain than in other organs. Endothelial cells of CNS blood vessels express particularly high levels of tight junction proteins as well as of substrate-selective transporters. Importantly, some regions of the CNS, including the so-called circumventricular organs, contain 'leaky' blood vessels and are not part of the brain side of the blood–brain barrier.
- Rostral migratory stream
-
(RMS). The RMS is a neurogenic pathway by which newly generated neurons migrate from the subventricular zone of the CNS towards the olfactory bulb; in rodents, this facilitates continuous replenishment of dying neurons of the olfactory system.
- Ectopic lymphoid tissues
-
Newly-formed lymphoid tissues arising at non-determined sites within affected tissues due to unresolved inflammatory processes. Much like secondary lymphoid tissues such as lymph nodes, spleen and Peyer's patches, ectopic (also known as tertiary) lymphoid organs harbour B cell follicles and support the induction and/or maintenance of adaptive immune responses. The formation of ectopic lymphoid tissues has been implicated in the pathogenesis of a wide range of chronic inflammatory conditions, including several autoimmune diseases.
- Pannus
-
Abnormal vessel-rich hypertrophic fibrovascular tissue developing from inflamed synovium within affected joints during rheumatoid arthritis. Owing to its infiltrative tumour-like growth, pannus formation leads to the progressive destruction of synovium, cartilage and bone during later stages of rheumatoid arthritis. The development of tertiary lymphoid tissue within the pannus has been described.
- slanDCs
-
Human DCs that are characterized by specific expression of the 6-sulfo N-acetyllactosamine (slan) instead of cutaneous lymphocyte antigen (CLA) or P-selectin glycoprotein ligand 1. SlanDCs are capable of producing very high levels of pro-inflammatory mediators and are associated with chronic inflammatory and autoimmune diseases of the skin.
Rights and permissions
About this article
Cite this article
Worbs, T., Hammerschmidt, S. & Förster, R. Dendritic cell migration in health and disease. Nat Rev Immunol 17, 30–48 (2017). https://doi.org/10.1038/nri.2016.116
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2016.116
This article is cited by
-
ANGPTL4 regulates ovarian cancer progression by activating the ERK1/2 pathway
Cancer Cell International (2024)
-
Differentiation, maturation, and collection of THP-1-derived dendritic cells based on a PEG hydrogel culture platform
Biotechnology Letters (2024)
-
Mass cytometry revealed the circulating immune cell landscape across different Suzuki stages of Moyamoya disease
Immunologic Research (2024)
-
Self-assembled peptide/polymer hybrid nanoplatform for cancer immunostimulating therapies
Drug Delivery and Translational Research (2024)
-
Identification of a non-canonical chemokine-receptor pathway suppressing regulatory T cells to drive atherosclerosis
Nature Cardiovascular Research (2024)