Key Points
-
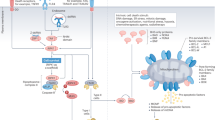
The immunogenicity of cell death is determined by its antigenicity and its adjuvanticity.
-
Cells infected by pathogens as well as cancer cells exhibit accrued antigenicity.
-
Stress responses in dying cells cause the emission of adjuvant-like danger signals.
-
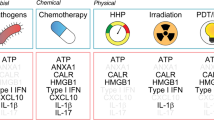
Different sets of danger signals are associated with distinct variants of immunogenic cell death.
-
Both pathogens and cancer cells interrupt danger signalling for their own benefit.
-
Reinstating the immunogenicity of cell death holds promise for anticancer therapy.
Abstract
Immunogenicity depends on two key factors: antigenicity and adjuvanticity. The presence of exogenous or mutated antigens explains why infected cells and malignant cells can initiate an adaptive immune response provided that the cells also emit adjuvant signals as a consequence of cellular stress and death. Several infectious pathogens have devised strategies to control cell death and limit the emission of danger signals from dying cells, thereby avoiding immune recognition. Similarly, cancer cells often escape immunosurveillance owing to defects in the molecular machinery that underlies the release of endogenous adjuvants. Here, we review current knowledge on the mechanisms that underlie the activation of immune responses against dying cells and their pathophysiological relevance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fuchs, Y. & Steller, H. Programmed cell death in animal development and disease. Cell 147, 742–758 (2011).
van Kempen, T. S., Wenink, M. H., Leijten, E. F., Radstake, T. R. & Boes, M. Perception of self: distinguishing autoimmunity from autoinflammation. Nat. Rev. Rheumatol. 11, 483–492 (2015).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Janeway, C. A. Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 13, 11–16 (1992).
Broz, P. & Monack, D. M. Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immunol. 13, 551–565 (2013).
Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 16, 35–50 (2016).
Chu, H. & Mazmanian, S. K. Innate immune recognition of the microbiota promotes host–microbial symbiosis. Nat. Immunol. 14, 668–675 (2013).
Fuchs, Y. & Steller, H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat. Rev. Mol. Cell Biol. 16, 329–344 (2015).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Bezu, L. et al. Combinatorial strategies for the induction of immunogenic cell death. Front. Immunol. 6, 187 (2015).
Galluzzi, L. et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 22, 58–73 (2015).
Kepp, O. et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 3, e955691 (2014).
Galluzzi, L., Brenner, C., Morselli, E., Touat, Z. & Kroemer, G. Viral control of mitochondrial apoptosis. PLoS Pathog. 4, e1000018 (2008).
LaRock, D. L., Chaudhary, A. & Miller, S. I. Salmonellae interactions with host processes. Nat. Rev. Microbiol. 13, 191–205 (2015).
Gay, N. J., Symmons, M. F., Gangloff, M. & Bryant, C. E. Assembly and localization of Toll-like receptor signalling complexes. Nat. Rev. Immunol. 14, 546–558 (2014).
Kanneganti, T. D. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10, 688–698 (2010).
Barber, G. N. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 35, 88–93 (2014).
Yoneyama, M., Onomoto, K., Jogi, M., Akaboshi, T. & Fujita, T. Viral RNA detection by RIG-I-like receptors. Curr. Opin. Immunol. 32, 48–53 (2015).
Sica, V. et al. Organelle-specific initiation of autophagy. Mol. Cell 59, 522–539 (2015).
Bettigole, S. E. & Glimcher, L. H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 33, 107–138 (2015).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O'Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Galluzzi, L. et al. Autophagy in malignant transformation and cancer progression. EMBO J. 34, 856–880 (2015).
Travassos, L. H. et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11, 55–62 (2010).
Liang, X. H. et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 72, 8586–8596 (1998).
Talloczy, Z. et al. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc. Natl Acad. Sci. USA 99, 190–195 (2002).
Roche, P. A. & Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 15, 203–216 (2015).
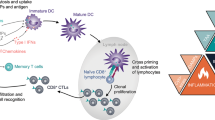
Joffre, O. P., Segura, E., Savina, A. & Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 12, 557–569 (2012).
Casares, N. et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202, 1691–1701 (2005). This is the first formal demonstration that anthracycline-treated mouse cancer cells can induce an adaptive immune response upon inoculation of immunocompetent syngeneic hosts in the absence of any adjuvant.
Dudek, A. M., Garg, A. D., Krysko, D. V., De Ruysscher, D. & Agostinis, P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 24, 319–333 (2013).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Panaretakis, T. et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 15, 1499–1509 (2008).
Fucikova, J. et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 71, 4821–4833 (2011).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011). This article provides robust evidence in support of the notion that autophagy is strictly required for mouse cancer cells succumbing to immunogenic chemotherapeutics to release amounts of ATP that are compatible with the engagement of adaptive immunity.
Sistigu, A. et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Vacchelli, E. et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 350, 972–978 (2015). This article demonstrates that chemotherapy-driven ICD depends on the interaction between ANXA1 released by dying cancer cells and FPR1 expressed by the host.
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009). This is the first demonstration that extracellular nucleotides including ATP and UTP generate a steep chemotactic gradient around dying cells, which guide the recruitment of myeloid cells through P2RY2.
Tesniere, A. et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 29, 482–491 (2010).
Martins, I. et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 30, 1147–1158 (2011).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Gubin, M. M. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014).
Van Allen, E. M. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015).
Garg, A. D., Krysko, D. V., Vandenabeele, P. & Agostinis, P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol. Immunother. 61, 215–221 (2012).
Fucikova, J. et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int. J. Cancer 135, 1165–1177 (2014). This is the first comprehensive molecular characterization of high hydrostatic pressure-driven ICD in human cancer cells.
Obeid, M. et al. Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC light-induced apoptosis. Cell Death Differ. 14, 1848–1850 (2007).
Galluzzi, L., Bravo- San Pedro, J. M., Kepp, O. & Kroemer, G. Regulated cell death and adaptive stress responses. Cell. Mol. Life Sci. 73, 2405–2410 (2016).
Goldszmid, R. S. et al. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J. Immunol. 171, 5940–5947 (2003).
Aaes, T. L. et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 15, 274–287 (2016).
Bloy, N. et al. Trial watch: dendritic cell-based anticancer therapy. Oncoimmunology 3, e963424 (2014).
Strome, S. E. et al. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res. 62, 1884–1889 (2002).
Kurokawa, T., Oelke, M. & Mackensen, A. Induction and clonal expansion of tumor-specific cytotoxic T lymphocytes from renal cell carcinoma patients after stimulation with autologous dendritic cells loaded with tumor cells. Int. J. Cancer 91, 749–756 (2001).
Gameiro, S. R. et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 5, 403–416 (2014).
Gorin, J. B. et al. Antitumor immunity induced after α irradiation. Neoplasia 16, 319–328 (2014).
Golden, E. B. et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 3, e28518 (2014).
Ko, A. et al. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 21, 92–99 (2014). This report discriminated for the first time between the cell-intrinsic and cell-extrinsic effects of autophagy in cancer cells responding to radiation therapy, unequivocally demonstrating that the latter dominate over the former in immunocompetent settings.
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Lim, J. Y., Gerber, S. A., Murphy, S. P. & Lord, E. M. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8+ T cells. Cancer Immunol. Immunother. 63, 259–271 (2014).
Brusa, D., Migliore, E., Garetto, S., Simone, M. & Matera, L. Immunogenicity of 56 °C and UVC-treated prostate cancer is associated with release of HSP70 and HMGB1 from necrotic cells. Prostate 69, 1343–1352 (2009).
Bernard, J. J. et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med. 18, 1286–1290 (2012).
Werthmöller, N., Frey, B., Wunderlich, R., Fietkau, R. & Gaipl, U. S. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis. 6, e1761 (2015).
Chen, L. C. et al. Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol. Med. 4, 1276–1293 (2012).
Demaria, S. et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 58, 862–870 (2004). This report demonstrates that increasing the availability of DCs is sufficient to induce robust abscopal effects in response to irradiation.
Golden, E. B., Demaria, S., Schiff, P. B., Chachoua, A. & Formenti, S. C. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 1, 365–372 (2013).
Vanpouille-Box, C., Pilones, K. A., Wennerberg, E., Formenti, S. C. & Demaria, S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine 33, 7415–7422 (2015).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Garg, A. D. et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 31, 1062–1079 (2012). This report characterizes the molecular mechanisms underlying CALR exposure and ATP secretion by mouse cancer cells exposed to hypericin-based PDT.
Korbelik, M., Zhang, W. & Merchant, S. Involvement of damage-associated molecular patterns in tumor response to photodynamic therapy: surface expression of calreticulin and high-mobility group box-1 release. Cancer Immunol. Immunother. 60, 1431–1437 (2011).
Weiss, E. M. et al. High hydrostatic pressure treatment generates inactivated mammalian tumor cells with immunogeneic features. J. Immunotoxicol. 7, 194–204 (2010).
Panaretakis, T. et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 28, 578–590 (2009).
Galluzzi, L., Kepp, O. & Kroemer, G. Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J. 31, 1055–1057 (2012).
Garg, A. D. et al. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy 9, 1292–1307 (2013). This paper shows that autophagy not only is dispensable for ATP release by cancer cells succumbing to hypericin-based PDT, but also limits CALR exposure by virtue of its antioxidant effects.
Wang, H. et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 (2014).
Dondelinger, Y. et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 7, 971–981 (2014).
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Yang, H. et al. Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology 5, e1149673 (2016). References 51 and 78 demonstrate for the first time that necroptosis can be perceived as immunogenic and hence induce an adaptive immune response.
Galluzzi, L., López-Soto, A., Kumar, S. & Kroemer, G. Caspases connect cell-death signaling to organismal homeostasis. Immunity 44, 221–231 (2016).
Galluzzi, L., Kepp, O. & Kroemer, G. Mitochondria: master regulators of danger signalling. Nat. Rev. Mol. Cell Biol. 13, 780–788 (2012).
Cekic, C. & Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 16, 177–192 (2016).
Hangai, S. et al. PGE2 induced in and released by dying cells functions as an inhibitory DAMP. Proc. Natl Acad. Sci. USA 113, 3844–3849 (2016).
Poon, I. K., Lucas, C. D., Rossi, A. G. & Ravichandran, K. S. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 14, 166–180 (2014).
Gardai, S. J. et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123, 321–334 (2005). This article identifies LRP1 as the main receptor that drives the uptake of dying cells exposing CALR on their membrane by myeloid cells.
Basu, S., Binder, R. J., Ramalingam, T. & Srivastava, P. K. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14, 303–313 (2001).
Lillis, A. P. et al. Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J. Immunol. 181, 364–373 (2008).
Cirone, M. et al. Primary effusion lymphoma cell death induced by bortezomib and AG 490 activates dendritic cells through CD91. PLoS ONE 7, e31732 (2012).
Gilardini Montani, M. S. et al. Capsaicin-mediated apoptosis of human bladder cancer cells activates dendritic cells via CD91. Nutrition 31, 578–581 (2015).
Garg, A. D. et al. Resistance to anticancer vaccination effect is controlled by a cancer cell-autonomous phenotype that disrupts immunogenic phagocytic removal. Oncotarget 6, 26841–26860 (2015).
Suzuki, S. et al. CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol. Rep. 28, 465–472 (2012).
Majeti, R. et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138, 286–299 (2009).
Wang, H. et al. Expression and significance of CD44, CD47 and c-met in ovarian clear cell carcinoma. Int. J. Mol. Sci. 16, 3391–3404 (2015).
Fucikova, J. et al. Calreticulin expression in human non-small cell lung cancers correlates with increased accumulation of antitumor immune cells and favorable prognosis. Cancer Res. 76, 1746–1756 (2016).
Stebbing, J. et al. The common heat shock protein receptor CD91 is up-regulated on monocytes of advanced melanoma slow progressors. Clin. Exp. Immunol. 138, 312–316 (2004).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Ma, Y. et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 38, 729–741 (2013).
Ma, Y. et al. Contribution of IL-17-producing γδ T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 208, 491–503 (2011).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Sims, G. P., Rowe, D. C., Rietdijk, S. T., Herbst, R. & Coyle, A. J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388 (2010).
Bergmann, C. et al. Toll-like receptor 4 single-nucleotide polymorphisms Asp299Gly and Thr399Ile in head and neck squamous cell carcinomas. J. Transl Med. 9, 139 (2011).
Tittarelli, A. et al. Toll-like receptor 4 gene polymorphism influences dendritic cell in vitro function and clinical outcomes in vaccinated melanoma patients. Cancer Immunol. Immunother. 61, 2067–2077 (2012).
Gast, A. et al. Association of inherited variation in Toll-like receptor genes with malignant melanoma susceptibility and survival. PLoS ONE 6, e24370 (2011).
Ladoire, S. et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy 11, 1878–1890 (2015).
Yamazaki, T. et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 21, 69–78 (2014).
Chiba, S. et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 13, 832–842 (2012).
Goritzka, M. et al. α/β interferon receptor signaling amplifies early proinflammatory cytokine production in the lung during respiratory syncytial virus infection. J. Virol. 88, 6128–6136 (2014).
Lazear, H. M. et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe 19, 720–730 (2016).
Robinson, N. et al. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat. Immunol. 13, 954–962 (2012).
Cervantes-Barragan, L. et al. Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection. J. Immunol. 182, 1099–1106 (2009).
Bidwell, B. N. et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 18, 1224–1231 (2012).
Chan, S. R. et al. STAT1-deficient mice spontaneously develop estrogen receptor α-positive luminal mammary carcinomas. Breast Cancer Res. 14, R16 (2012).
Salaun, B. et al. TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res. 71, 1607–1614 (2011).
Perretti, M. & D'Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70 (2009).
Leib, D. A., Alexander, D. E., Cox, D., Yin, J. & Ferguson, T. A. Interaction of ICP34.5 with beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J. Virol. 83, 12164–12171 (2009).
Galluzzi, L. et al. Viral strategies for the evasion of immunogenic cell death. J. Intern. Med. 267, 526–542 (2010).
Kepp, O. et al. Viral subversion of immunogenic cell death. Cell Cycle 8, 860–869 (2009).
Taxman, D. J., Huang, M. T. & Ting, J. P. Inflammasome inhibition as a pathogenic stealth mechanism. Cell Host Microbe 8, 7–11 (2010).
Galluzzi, L., Kepp, O., Chan, F. K. & Kroemer, G. Necroptosis: mechanisms and relevance to disease. Annu. Rev. Pathol. (in the press).
Cordano, P. et al. The E6E7 oncoproteins of cutaneous human papillomavirus type 38 interfere with the interferon pathway. Virology 377, 408–418 (2008).
Mittal, D., Gubin, M. M., Schreiber, R. D. & Smyth, M. J. New insights into cancer immunoediting and its three component phases — elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Li, M. O. & Rudensky, A. Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 16, 220–233 (2016).
Fucikova, J. et al. Prognostic and predictive value of DAMPs and DAMP-associated processes in cancer. Front. Immunol. 6, 402 (2015).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28, 690–714 (2015).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Ni, M. et al. Serum levels of calreticulin in correlation with disease activity in patients with rheumatoid arthritis. J. Clin. Immunol. 33, 947–953 (2013).
Sanchez, D. et al. Occurrence of IgA and IgG autoantibodies to calreticulin in coeliac disease and various autoimmune diseases. J. Autoimmun. 15, 441–449 (2000).
Zhou, Z. et al. Immunoproteomic to identify antigens in the intestinal mucosa of Crohn's disease patients. PLoS ONE 8, e81662 (2013).
Menger, L. et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci. Transl Med. 4, 143ra99 (2012).
van der Burg, S. H., Arens, R., Ossendorp, F., van Hall, T. & Melief, C. J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 16, 219–233 (2016).
Acknowledgements
The authors are supported by the French Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Institut Universitaire de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LeDucq Foundation; the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Microorganism-associated molecular patterns
-
(MAMPs). Conserved microbial components that, upon detection by the host, can favour the establishment of immunological tolerance or promote a state of accrued resistance to infection.
- Damage-associated molecular patterns
-
(DAMPs). Endogenous molecules that are normally invisible to the host immune system but, once emitted by stressed or dying cells, initiate danger signalling.
- Checkpoint blockers
-
Clinically used monoclonal antibodies that instate (or reinstate) anticancer immunosurveillance by inhibiting immunosuppressive receptors like cytotoxic T lymphocyte associated protein 4 (CTLA4) or programmed cell death 1 (PDCD1; also known as PD1).
- Unfolded protein response
-
(UPR). Stress-responsive mechanism that increases the ability of the endoplasmic reticulum to cope with an increased load of unfolded polypeptides.
- Cytosolic DNA sensors
-
Intracellular PRRs including Z-DNA binding protein 1 (ZBP1; also known as DAI) that are involved in the detection of cytosolic double-stranded DNA.
- RIG-I-like receptors
-
Intracellular PRRs resembling DEAD box protein 58 (DDX58; also known as RIG-I) that are involved in the detection of cytosolic double-stranded RNA.
- NOD-like receptors
-
Intracellular PRRs involved in the detection of a wide panel of MAMPs of both bacterial and viral origin.
- Inflammasome
-
Large supramolecular platform responsible for the activation of caspase 1 and consequent proteolytic maturation of IL-1β and IL-18.
- Photodynamic therapy
-
(PDT). A treatment for pre-malignant and malignant skin conditions, based on the administration of a drug that operates as a photosensitizer followed by exposure to a particular type of light.
- Accidental necrosis
-
A form of cell death that cannot be modulated by pharmacological or genetic interventions, invariably manifesting with necrotic morphological features.
- Abscopal effect
-
Immunological response to radiation therapy whereby the irradiation of a malignant lesion results in the regression or stabilization of a distant, non-irradiated lesion.
- Z-VAD-fmk
-
A chemical agent that inhibits a wide panel of proteolytic enzymes, including several caspases and calpains.
- SMAC mimetic
-
A chemical agent that resembles second mitochondria-derived activator of caspase (SMAC; also known as DIABLO) in its ability to inhibit various members of the inhibitor of apoptosis (IAP) protein family.
- BCL-2 protein family
-
A large group of proteins sharing one to four B cell lymphoma 2 (BCL-2) homology (BH) domains, which play a crucial role in the regulation of some variants of apoptotic cell death.
- Necrosome
-
An amyloid-like supramolecular platform that precipitates necroptosis by allowing for the physical and functional interaction between RIPK1, RIPK3 and MLKL.
- Antigen spreading
-
Immunological phenomenon whereby the antigenic targets of an adaptive immune response expand and diversify over time, presumably as a consequence of sustained cell death and DAMP signalling.
Rights and permissions
About this article
Cite this article
Galluzzi, L., Buqué, A., Kepp, O. et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17, 97–111 (2017). https://doi.org/10.1038/nri.2016.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2016.107
This article is cited by
-
Forefronts and hotspots evolution of the nanomaterial application in anti-tumor immunotherapy: a scientometric analysis
Journal of Nanobiotechnology (2024)
-
Crosstalk between metabolism and cell death in tumorigenesis
Molecular Cancer (2024)
-
Immune characteristics and clinical significance of peripheral blood lymphocytes in breast cancer
BMC Cancer (2024)
-
Neoadjuvant chemo-immunotherapy with camrelizumab plus nab-paclitaxel and cisplatin in resectable locally advanced squamous cell carcinoma of the head and neck: a pilot phase II trial
Nature Communications (2024)
-
Chemokine-derived oncolytic peptide induces immunogenic cancer cell death and significantly suppresses tumor growth
Cell Death Discovery (2024)