Key Points
-
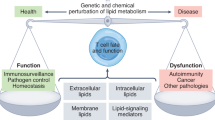
Lipid molecules are extremely diverse, but their functions are poorly understood. Different lipid species are co-regulated in immune cells.
-
Lipids have heterogeneous distributions in the membrane bilayer, and they form various lipid domains to provide platforms for local signalling.
-
Different T cell subsets have distinct membrane lipid compositions, which are crucial for their physiological functions.
-
Membrane lipids have sophisticated roles in regulating T cell signalling — such as formation of the immune synapse, recruitment of cytosolic signalling proteins, safety control of immunoreceptors, mediating protein island formation and regulating the conformation, partitioning and mobility of membrane proteins. A single lipid molecule can have multiple regulatory functions.
-
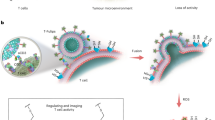
Modulating membrane lipid composition of T cells can be applied to treat cancers and autoimmune diseases. Cellular lipid metabolic pathways have 'metabolic checkpoints' that are good drug targets for immunotherapies.
-
More mechanistic studies on membrane lipids are needed to pave the way for the development of new generation of lipid-based immunotherapies.
Abstract
The plasma membrane is an essential cellular structure that separates the cell interior from the extracellular environment, while allowing for the exchange of signals and materials that are essential for cell survival and function. The complexity of the lipids in the plasma membrane has been long appreciated, but recent developments in lipidomics and imaging technologies have improved our understanding of plasma membrane lipid dynamics. New studies have started to unveil important functions for plasma membrane lipids in regulating T cell signalling. Importantly, it has been shown that the modulation of membrane lipids can be used to harness T cell activity to treat cancer and autoimmunity. Therefore, lipid-based immunotherapy might be a promising new clinical strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
12 February 2018
In the main text of this Review article, ACAT1 was incorrectly defined as 'acetyl-CoA acetyltransferase, mitochondrial'. The correct definition of ACAT1 in this article is 'acyl-CoA cholesterol acyltransferase 1'. Nature Reviews Immunology apologize for this error, which has now been corrected in the PDF and HTML versions of the article.
References
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Lingwood, D. & Simons, K. Lipid rafts as a membrane-organizing principle. Science 327, 46–50 (2010).
Brugger, B. Lipidomics: analysis of the lipid composition of cells and subcellular organelles by electrospray ionization mass spectrometry. Annu. Rev. Biochem. 83, 79–98 (2014).
Koberlin, M. S. et al. A conserved circular network of coregulated lipids modulates innate immune responses. Cell 162, 170–183 (2015). This study used lipodomics and genetics tools to show that different lipid species are co-regulated in immune cells and that lipid metabolism directly regulates innate immune responses.
Coskun, U. & Simons, K. Cell membranes: the lipid perspective. Structure 19, 1543–1548 (2011).
Merrill, A. H. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111, 6387–6422 (2011).
Chang, T. Y., Chang, C. C., Ohgami, N. & Yamauchi, Y. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22, 129–157 (2006).
Fadeel, B. & Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 44, 264–277 (2009).
van den Bogaart, G. et al. Membrane protein sequestering by ionic protein-lipid interactions. Nature 479, 552–555 (2011). This study shows that acidic phospholipids can form nanoclusters on the plasma membrane and ionic protein–lipid interactions have an important role in mediating the lipid domain formation.
Raghupathy, R. et al. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 161, 581–594 (2015).
Eggeling, C. et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457, 1159–1162 (2009). This study used stimulated emission depletion (STED) far-field fluorescence nanoscopy to directly detect single-lipid diffusion in the plasma membrane of living cells.
Honigmann, A. et al. Scanning STED-FCS reveals spatiotemporal heterogeneity of lipid interaction in the plasma membrane of living cells. Nat. Commun. 5, 5412 (2014).
Mueller, V. et al. STED nanoscopy reveals molecular details of cholesterol- and cytoskeleton-modulated lipid interactions in living cells. Biophys. J. 101, 1651–1660 (2011).
Vicidomini, G. et al. STED-FLCS: an advanced tool to reveal spatiotemporal heterogeneity of molecular membrane dynamics. Nano Lett. 15, 5912–5918 (2015).
Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Bio. 9, 125–138 (2008).
Garg, S., Tang, J. X., Ruhe, J. & Naumann, C. A. Actin-induced perturbation of PS lipid-cholesterol interaction: a possible mechanism of cytoskeleton-based regulation of membrane organization. J. Struct. Biol. 168, 11–20 (2009).
Zhou, Y. et al. Signal transduction. Membrane potential modulates plasma membrane phospholipid dynamics and K-Ras signaling. Science 349, 873–876 (2015). This study shows that membrane potential can directly affect the clustering of certain species of acidic phospholipids and consequently affect K-RAS signalling.
Boettcher, J. M. et al. Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry 50, 2264–2273 (2011).
Levental, I. et al. Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers. Biochemistry 48, 8241–8248 (2009).
Milovanovic, D. et al. Calcium promotes the formation of syntaxin 1 mesoscale domains through phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 291, 7868–7876 (2016).
Wang, Y. H. et al. Divalent cation-induced cluster formation by polyphosphoinositides in model membranes. J. Am. Chem. Soc. 134, 3387–3395 (2012).
Shi, X. et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature 493, 111–115 (2013). This study shows that Ca2+ can directly bind to the phosphate group of acidic phospholipid to neutralize the negative charge, which leads to the disruption of the ionic interaction between CD3 cytoplasmic domains and acidic phospholipid and the subsequent exposure of CD3 tyrosine sites for phosphorylation.
Bensinger, S. J. et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134, 97–111 (2008). This study shows that cholesterol metabolism of T cells is fully reprogrammed upon cell activation to support cell proliferation.
Kidani, Y. et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 14, 489–499 (2013).
Yang, W. et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016). This study shows that the cholesterol esterification enzyme ACAT1 is a metabolic checkpoint in CD8+ T cells and inhibition of its activity leads to potentiated antitumor T cell immunity.
Fischer, K. et al. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood 108, 4094–4101 (2006).
Gagnon, E., Schubert, D. A., Gordo, S., Chu, H. H. & Wucherpfennig, K. W. Local changes in lipid environment of TCR microclusters regulate membrane binding by the CD3ɛ cytoplasmic domain. J. Exp. Med. 209, 2423–2439 (2012).
Cho, J. H., Kim, H. O., Surh, C. D. & Sprent, J. T. Cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity 32, 214–226 (2010).
Frechin, M. et al. Cell-intrinsic adaptation of lipid composition to local crowding drives social behaviour. Nature 523, 88–91 (2015).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Moravcevic, K., Oxley, C. L. & Lemmon, M. A. Conditional peripheral membrane proteins: facing up to limited specificity. Structure 20, 15–27 (2012).
Li, L. Y., Shi, X. S., Guo, X. D., Li, H. & Xu, C. Q. Ionic protein lipid interaction at the plasma membrane: what can the charge do? Trends Biochem. Sci. 39, 130–140 (2014).
Contreras, F. X. et al. Molecular recognition of a single sphingolipid species by a protein's transmembrane domain. Nature 481, 525–529 (2012).
Bjorkholm, P. et al. Identification of novel sphingolipid-binding motifs in mammalian membrane proteins. Biochim. Biophys. Acta 1838, 2066–2070 (2014).
Li, H. & Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 139, 4991–4997 (1998).
Fantini, J., Di Scala, C., Evans, L. S., Williamson, P. T. & Barrantes, F. J. A mirror code for protein-cholesterol interactions in the two leaflets of biological membranes. Scientif. Rep. 6, 21907 (2016).
Sever, N. et al. Insig-dependent ubiquitination and degradation of mammalian 3-hydroxy-3-methylglutaryl-CoA reductase stimulated by sterols and geranylgeraniol. J. Biol. Chem. 278, 52479–52490 (2003).
Hanson, M. A. et al. A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure 16, 897–905 (2008).
Landreh, M., Marty, M. T., Gault, J. & Robinson, C. V. A sliding selectivity scale for lipid binding to membrane proteins. Curr. Opin. Struct. Biol. 39, 54–60 (2016).
Dustin, M. L. The immunological synapse. Cancer Immunol. Res. 2, 1023–1033 (2014).
Gaus, K., Chklovskaia, E., Fazekas de St Groth, B., Jessup, W. & Harder, T. Condensation of the plasma membrane at the site of T lymphocyte activation. J. Cell Biol. 171, 121–131 (2005).
Rentero, C. et al. Functional implications of plasma membrane condensation for T cell activation. PLoS ONE 3, e2262 (2008).
Zech, T. et al. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 28, 466–476 (2009).
Owen, D. M. et al. High plasma membrane lipid order imaged at the immunological synapse periphery in live T cells. Mol. Membr. Biol. 27, 178–189 (2010).
Miguel, L. et al. Primary human CD4+ T cells have diverse levels of membrane lipid order that correlate with their function. J. Immunol. 186, 3505–3516 (2011).
Kallikourdis, M. et al. Phosphatidylinositol 4-phosphate 5-kinase β controls recruitment of lipid rafts into the immunological synapse. J. Immunol. 196, 1955–1963 (2016).
Tavano, R. et al. CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunological synapse. Nat. Cell Biol. 8, 1270–1276 (2006).
Tavano, R. et al. CD28 and lipid rafts coordinate recruitment of Lck to the immunological synapse of human T lymphocytes. J. Immunol. 173, 5392–5397 (2004).
Viola, A., Schroeder, S., Sakakibara, Y. & Lanzavecchia, A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283, 680–682 (1999).
Quann, E. J., Merino, E., Furuta, T. & Huse, M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat. Immunol. 10, 627–635 (2009).
Spitaler, M., Emslie, E., Wood, C. D. & Cantrell, D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity 24, 535–546 (2006).
Ritter, A. T. et al. Actin depletion initiates events leading to granule secretion at the immunological synapse. Immunity 42, 864–876 (2015).
Chauveau, A., Le Floc'h, A., Bantilan, N. S., Koretzky, G. A. & Huse, M. Diacylglycerol kinase alpha establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci. Signal. 7, ra82 (2014).
Liu, X., Kapoor, T. M., Chen, J. K. & Huse, M. Diacylglycerol promotes centrosome polarization in T cells via reciprocal localization of dynein and myosin II. Proc. Natl Acad. Sci. USA 110, 11976–11981 (2013).
Quann, E. J., Liu, X., Altan-Bonnet, G. & Huse, M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat. Immunol. 12, 647–654 (2011).
Le Floc'h, A. et al. Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. J. Exp. Med. 210, 2721–2737 (2013).
Costello, P. S., Gallagher, M. & Cantrell, D. A. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat. Immunol. 3, 1082–1089 (2002).
Harriague, J. & Bismuth, G. Imaging antigen-induced PI3K activation in T cells. Nat. Immunol. 3, 1090–1096 (2002).
Smith-Garvin, J. E., Koretzky, G. A. & Jordan, M. S. T cell activation. Annu. Rev. Immunol. 27, 591–619 (2009).
Heo, W. D. et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314, 1458–1461 (2006). This study shows that ionic protein–lipid interactions can target cytosolic proteins to the plasma membrane.
Walsh, C. M. et al. Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem. J. 425, 159–168 (2010).
Park, M. J. et al. SH2 domains serve as lipid-binding modules for pTyr-signaling proteins. Mol. Cell 62, 7–20 (2016). This study shows that SH2 domains have acidic phospholipid binding sites in addition to the well-known phosphorylated-Tyr binding sites.
Nika, K. et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32, 766–777 (2010).
Wu, W. et al. Lipid in T-cell receptor transmembrane signaling. Prog. Biophys. Mol. Biol. 118, 130–138 (2015).
DeFord-Watts, L. M. et al. The CD3 ζ subunit contains a phosphoinositide-binding motif that is required for the stable accumulation of TCR-CD3 complex at the immunological synapse. J. Immunol. 186, 6839–6847 (2011).
DeFord-Watts, L. M. et al. The cytoplasmic tail of the T cell receptor CD3 ɛ subunit contains a phospholipid-binding motif that regulates T cell functions. J. Immunol. 183, 1055–1064 (2009).
Xu, C. et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3ɛ cytoplasmic tyrosine-based motif. Cell 135, 702–713 (2008). This study shows that acidic phospholipids can ionically interact with the cytoplasmic domain of CD3 chain to sequester the key tyrosines within the membrane, thereby providing a safety control on T cell receptor activity.
Aivazian, D. & Stern, L. J. Phosphorylation of T cell receptor ζ is regulated by a lipid dependent folding transition. Nat. Struct. Biol. 7, 1023–1026 (2000).
Zhang, H., Cordoba, S. P., Dushek, O. & van der Merwe, P. A. Basic residues in the T-cell receptor ζ cytoplasmic domain mediate membrane association and modulate signaling. Proc. Natl Acad. Sci. USA 108, 19323–19328 (2011).
Chen, X. et al. Acidic phospholipids govern the enhanced activation of IgG-B cell receptor. Nat. Commun. 6, 8552 (2015).
Lillemeier, B. F. et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat. Immunol. 11, 90–96 (2010); erratum 11, 543.
Sherman, E. et al. Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity 35, 705–720 (2011).
Roh, K. H., Lillemeier, B. F., Wang, F. & Davis, M. M. The coreceptor CD4 is expressed in distinct nanoclusters and does not colocalize with T-cell receptor and active protein tyrosine kinase p56lck. Proc. Natl Acad. Sci. USA 112, E1604–E1613 (2015).
Rossy, J., Owen, D. M., Williamson, D. J., Yang, Z. & Gaus, K. Conformational states of the kinase Lck regulate clustering in early T cell signaling. Nat. Immunol. 14, 82–89 (2013).
Kumar, R. et al. Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes. Immunity 35, 375–387 (2011).
Molnar, E. et al. Cholesterol and sphingomyelin drive ligand-independent T-cell antigen receptor nanoclustering. J. Biol. Chem. 287, 42664–42674 (2012).
Wang, F., Beck-Garcia, K., Zorzin, C., Schamel, W. W. & Davis, M. M. Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. 17, 844–850 Nat. Immunol. (2016).
Owen, D. M., Williamson, D. J., Magenau, A. & Gaus, K. Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat. Commun. 3, 1256 (2012).
Su, X. et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 (2016).
Davis, S. J. & van der Merwe, P. A. The kinetic-segregation model: TCR triggering and beyond. Nat. Immunol. 7, 803–809 (2006).
Dawaliby, R. et al. Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat. Chem. Biol. 12, 35–39 (2016).
Hansen, S. B., Tao, X. & MacKinnon, R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 (2011).
Swamy, M. et al. A cholesterol-based allostery model of T cell receptor phosphorylation. Immunity 44, 1091–1101 (2016).
Coskun, U., Grzybek, M., Drechsel, D. & Simons, K. Regulation of human EGF receptor by lipids. Proc. Natl Acad. Sci. USA 108, 9044–9048 (2011).
Kabouridis, P. S. & Jury, E. C. Lipid rafts and T-lymphocyte function: implications for autoimmunity. FEBS Lett. 582, 3711–3718 (2008).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Tardif, J. C. et al. Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation 110, 3372–3377 (2004).
Moulton, V. R. & Tsokos, G. C. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J. Clin. Invest. 125, 2220–2227 (2015).
Jury, E. C., Kabouridis, P. S., Flores-Borja, F., Mageed, R. A. & Isenberg, D. A. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J. Clin. Invest. 113, 1176–1187 (2004).
Krishnan, S. et al. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J. Immunol. 172, 7821–7831 (2004).
McDonald, G. et al. Normalizing glycosphingolipids restores function in CD4+ T cells from lupus patients. J. Clin. Invest. 124, 712–724 (2014). This study shows that normalizing glycosphingolipid metabolism can correct functional defects of CD4+ T cells isolated from patients with SLE.
Jury, E. C., Isenberg, D. A., Mauri, C. & Ehrenstein, M. R. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. J. Immunol. 177, 7416–7422 (2006).
Butters, T. D., Dwek, R. A. & Platt, F. M. Imino sugar inhibitors for treating the lysosomal glycosphingolipidoses. Glycobiology 15, R43–R52 (2005).
Zhu, Y. X. et al. Lowering glycosphingolipid levels in CD4+ T cells attenuates T cell receptor signaling, cytokine production, and differentiation to the Th17 lineage. J. Biol. Chem. 286, 14787–14794 (2011).
Deng, G. M. & Tsokos, G. C. Cholera toxin B accelerates disease progression in lupus-prone mice by promoting lipid raft aggregation. J. Immunol. 181, 4019–4026 (2008).
Belluzzi, A. et al. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. New Engl. J. Med. 334, 1557–1560 (1996).
Farzaneh-Far, R., Harris, W. S., Garg, S., Na, B. & Whooley, M. A. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis 205, 538–543 (2009).
Uchiyama, K. et al. N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm. Bowel Dis. 16, 1696–1707 (2010).
Calder, P. C. & Newsholme, E. A. Polyunsaturated fatty-acids suppress human peripheral-blood lymphocyte-proliferation and interleukin-2 production. Clin. Sci. 82, 695–700 (1992).
Petursdottir, D. H. & Hardardottir, I. Dietary fish oil decreases secretion of T helper (Th) 1-type cytokines by a direct effect on murine splenic T cells but enhances secretion of a Th2-type cytokine by an effect on accessory cells. Brit. J. Nutr. 101, 1040–1046 (2009).
Allen, M. J. et al. n-3 PUFAs reduce T-helper 17 cell differentiation by decreasing responsiveness to interleukin-6 in isolated mouse splenic CD4+ T cells. J. Nutr. 144, 1306–1313 (2014).
Monk, J. M., Hou, T. Y., Turk, H. F., McMurray, D. N. & Chapkin, R. S. n3 PUFAs reduce mouse CD4+ T-Cell ex vivo polarization into Th17 cells. J. Nutr. 143, 1501–1508 (2013).
Monk, J. M. et al. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J. Nutr. 142, 117–124 (2012).
Fan, Y. Y., Ly, L. H., Barhoumi, R., McMurray, D. N. & Chapkin, R. S. Dietary docosahexaenoic acid suppresses T cell protein kinase Cθ lipid raft recruitment and IL-2 production. J. Immunol. 173, 6151–6160 (2004).
Fan, Y. Y., McMurray, D. N., Ly, L. H. & Chapkin, R. S. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J. Nutr. 133, 1913–1920 (2003).
Hou, T. Y., McMurray, D. N. & Chapkin, R. S. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur. J. Pharmacol. (2015).
Hou, T. Y. et al. n-3 polyunsaturated fatty acids suppress phosphatidylinositol 4,5-bisphosphate-dependent actin remodelling during CD4+ T-cell activation. Biochem. J. 443, 27–37 (2012).
Hou, T. Y. et al. n-3 polyunsaturated fatty acids suppress CD4+ T cell proliferation by altering phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] organization. Biochim. Biophys. Acta 1858, 85–96 (2016).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015).
Mourelle, M., Guarner, F. & Malagelada, J. R. Polyunsaturated phosphatidylcholine prevents stricture formation in a rat model of colitis. Gastroenterology 110, 1093–1097 (1996).
van Dieren, J. M. et al. Anti-inflammatory actions of phosphatidylinositol. Eur. J. Immunol. 41, 1047–1057 (2011).
Verhaar, A. P. et al. Miltefosine suppresses inflammation in a mouse model of inflammatory bowel disease. Inflamm. Bowel Dis. 19, 1974–1982 (2013).
Baumer, W., Wlaz, P., Jennings, G. & Rundfeldt, C. The putative lipid raft modulator miltefosine displays immunomodulatory action in T-cell dependent dermal inflammation models. Eur. J. Pharmacol. 628, 226–232 (2010).
Acknowledgements
C.X. is funded by CAS Strategic Priority Research Program XDB08020100, NSFC (31370860, 31425009 and 31530022) and MOST (2012CB910804). The authors thank C. Yan and J. Guo for their assistance in the preparation of this manuscript. We apologize to the many scientists whose work could not be included into this review owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Lipid raft model
-
The lipid raft model was initially developed to describe the membrane domains that are resistant to cold detergent extraction or mechanical disruption. Now, lipid rafts are viewed as dynamic nanoscale assemblies of sphingolipids, cholesterol and proteins that support local membrane signalling and trafficking.
- Free diffusion mode
-
Molecules diffuse freely in Brownian motion.
- Trapped diffusion mode
-
Molecules do not diffuse freely but can be transiently trapped into domains of higher molecular order when interacting with immobilized or slow moving entities.
- Ferroptosis
-
A non-apoptotic form of cell death that is defined by the iron-dependent accumulation of lipid reactive oxygen species and depletion of plasma membrane polyunsaturated fatty acids.
- Palmitoylation sites
-
Cysteine sites for reversible post-translational addition of palmitic acid to the sulfhydryl group to form a thioester. Palmitoylation regulates protein trafficking, sorting, clustering, stability and aggregation.
Rights and permissions
About this article
Cite this article
Wu, W., Shi, X. & Xu, C. Regulation of T cell signalling by membrane lipids. Nat Rev Immunol 16, 690–701 (2016). https://doi.org/10.1038/nri.2016.103
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2016.103
This article is cited by
-
A simple and rapid extraction of lipids in plasma using spin column with superabsorbent polymer beads for mass spectrometry
Journal of Analytical Science and Technology (2023)
-
Lipid scrambling in immunology: why it is important
Cellular & Molecular Immunology (2023)
-
Effects of high-fat diet on thyroid autoimmunity in the female rat
BMC Endocrine Disorders (2022)
-
The interplay between membrane topology and mechanical forces in regulating T cell receptor activity
Communications Biology (2022)
-
Developing Nanodisc-ID for label-free characterizations of membrane proteins
Communications Biology (2021)