Key Points
-
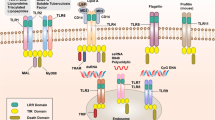
Several regulators and amplifiers are involved in the fine-tuning of pattern-recognition receptor (PRR) signalling via distinct self-regulation mechanisms.
-
Several distinct modes and new mechanistic insights of cross-regulation of PRR-driven responses have been highlighted by recent research.
-
A complicated interplay between PRRs and/or other immune pathways orchestrates the outcome of host immune defences through synergy, enhancement, suppression, feedback enhancement and feedback suppression.
-
Some epigenetic molecules, post-translational modification enzymes and metabolic mediators regulate innate signalling in an inducible manner, and many of these signalling pathways are involved in immune disorders.
-
Dysfunction of PRR signalling owing to dysregulated regulatory mechanisms is associated with the pathogenesis and prognosis of many immunological diseases.
-
There remain several challenging issues to address, from uncovering the specificity and selectivity of PRR-mediated responses, the assembly of the innate sensor and effector machinery to the targeting of crucial molecules involved in PRR signalling for the control of immunological diseases.
Abstract
In the initiation of innate immune responses against pathogens, pattern-recognition receptors (PRRs) have an essential role in recognizing specific components of microorganisms and triggering responses that eliminate the invading microorganisms. However, inappropriate activation of PRRs can lead to prolonged inflammation and even to autoimmune and inflammatory diseases. Thus, PRR-triggered responses are regulated through the degradation or translocation of the innate receptors themselves and through the involvement of intracellular regulators or amplifiers. In addition, a complex interplay between PRRs and/or other immune pathways finely tunes the outcome of host immune defence responses. In this Review, I describe many of the numerous distinct mechanisms for the self-regulation and cross-regulation of innate immune receptor signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Broz, P. & Monack, D. M. Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immunol. 13, 551–565 (2013).
Qian, C., Liu, J. & Cao, X. Innate signaling in the inflammatory immune disorders. Cytokine Growth Factor Rev. 25, 731–773 (2014).
O'Neill, L. A., Golenbock, D. & Bowie, A. G. The history of Toll-like receptors — redefining innate immunity. Nat. Rev. Immunol. 13, 453–460 (2013).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
DiDonato, J. A., Mercurio, F. & Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 246, 379–400 (2012).
Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Philpott, D. et al. NOD proteins: regulators of inflammation in health and disease. Nat. Rev. Immunol. 14, 9–23 (2014).
Carpenter, S., Ricci, E. P., Mercier, B. C., Moore, M. J. & Fitzgerald, K. A. Post-transcriptional regulation of gene expression in innate immunity. Nat. Rev. Immunol. 14, 361–376 (2014).
Zanoni, I. et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147, 868–880 (2011).
Wang, Y. et al. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood 110, 962–971 (2007).
Chuang, T. H. & Ulevitch, R. J. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat. Immunol. 5, 495–502 (2004). This study establishes the role of TRIAD3A in the ubiquitylation and proteolytic degradation of TLR4 and TLR9.
Park, B. et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 9, 1407–1414 (2008).
Ewald, S. E. et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456, 658–662 (2008).
Arimoto, K. et al. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc. Natl Acad. Sci. USA 104, 7500–7505 (2007).
Chen, W. et al. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell 152, 467–478 (2013). This paper reports the feedback regulation of RIG-I homeostasis and antiviral responses by Siglec-G.
Brinkmann, M. M. et al. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 177, 265–275 (2007).
Kim, Y. M., Brinkmann, M. M., Paquet, M. E. & Ploegh, H. L. UNC93B1 delivers nucleotide-sensing Toll-like receptors to endolysosomes. Nature 452, 234–238 (2008). References 18 and 19 reveal a crucial role for UNC93B1 in delivering TLR3, TLR7 and TLR9 to endolysosomes, where downstream signalling is initiated.
Fukui, R. et al. Unc93B1 biases Toll-like receptor responses to nucleic acid in dendritic cells toward DNA- but against RNA-sensing. J. Exp. Med. 206, 1339–1350 (2009).
Fukui, R. et al. Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity 35, 69–81 (2011).
Yang, Y. et al. Heat shock protein gp96 is a master chaperone for Toll-like receptors and is important in the innate function of macrophages. Immunity 26, 215–226 (2007).
Takahashi, K. et al. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J. Exp. Med. 204, 2963–2976 (2007).
da Silva Correia. J., Soldau, K., Christen, U., Tobias, P. S. & Ulevitch, R. J. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 276, 21129–21135 (2001).
Lee, H. K., Dunzendorfer, S., Soldau, K. & Tobias, P. S. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity 24, 153–163 (2006).
Baumann, C. L. et al. CD14 is a coreceptor of Toll-like receptors 7 and 9. J. Exp. Med. 207, 2689–2701 (2010).
Wang, D. et al. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc. Natl Acad. Sci. USA 107, 13806–13811 (2010).
Álvarez-Errico, D., Vento-Tormo, R., Sieweke, M. & Ballestar, E. Epigenetic control of myeloid cell differentiation, identity and function. Nat. Rev. Immunol. 15, 7–17 (2015).
Zhang, Q. et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 (2105).
Feng, J. et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 13, 423–430 (2010).
Feinberg, A. P. Phenotypic plasticity and the epigenetics of human disease. Nature 447, 433–440 (2007).
Allis, C. D. et al. New nomenclature for chromatin-modifying enzymes. Cell 131, 633–636 (2007).
Austenaa, L. et al. The histone methyltransferase Wbp7 controls macrophage function through GPI glycolipid anchor synthesis. Immunity 36, 572–585 (2012).
Wang, X. et al. MLL1, a H3K4 methyltransferase, regulates the TNFα-stimulated activation of genes downstream of NF-κB. J. Cell Sci. 125, 4058–4066 (2012).
De Santa, F. et al. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 130, 1083–1094 (2007).
Satoh, T. et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 11, 936–944 (2010). This paper shows that JMJD3-mediated H3K27 demethylation is crucial for regulating M2 macrophage development and host defence against helminth infection.
Fang, T. C. et al. Histone H3 lysine 9 di-methylation as an epigenetic signature of the interferon response. J. Exp. Med. 209, 661–669 (2012).
Schliehe, C. et al. The methyltransferase Setdb2 mediates virus-induced susceptibility to bacterial superinfection. Nat. Immunol. 16, 67–74 (2015).
Liu, Y. et al. Histone lysine methyltransferase Ezh1 promotes TLR-triggered inflammatory cytokine production by suppressing Tollip. J. Immunol. 194, 2838–2846 (2015).
Xia, M. et al. Histone methyltransferase Ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme A20. Immunity 39, 470–481 (2013).
Hammer, G. E. et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat. Immunol. 12, 1184–1193 (2011).
Boone, D. L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 (2004).
Shembade, N., Ma, A. & Harhaj, E. W. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 (2010). References 42 and 43 establish a crucial role for A20 in regulating TLR signalling and maintaining immune homeostasis.
Yang, X. J. & Seto, E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26, 5310–5318 (2007).
Kawahara, T. L. et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 136, 62–74 (2009).
Sadler, A. J. et al. BTB-ZF transcriptional regulator PLZF modifies chromatin to restrain inflammatory signaling programs. Proc. Natl Acad. Sci. USA 112, 1535–1540 (2015).
Zhou, W. et al. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Mol. Cell 29, 69–80 (2008).
Turner, M., Galloway, A. & Vigorito, E. Noncoding RNA and its associated proteins as regulatory elements of the immune system. Nat. Immunol. 15, 484–491 (2014).
Carpenter, S. et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science 341, 789–792 (2013). This paper reports that lincRNA- Cox2 activates or suppresses the transcription of distinct sets of inflammatory genes.
Rapicavoli, N. A. et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. eLife 2, e00762 (2013).
Li, Z. et al. The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proc. Natl Acad. Sci. USA 111, 1002–1007 (2014).
Wang, P. et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 344, 310–313 (2014).
Mowen, K. A. & David, M. Unconventional post-translational modifications in immunological signaling. Nat. Immunol. 15, 512–520 (2014).
Gack, M. U. et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, 916–920 (2007).
Oshiumi, H., Matsumoto, M., Hatakeyama, S. & Seya, T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-β induction during the early phase of viral infection. J. Biol. Chem. 284, 807–817 (2009).
Friedman, C. S. et al. The tumor suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Rep. 9, 930–936 (2008).
Zeng, W. et al. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 141, 315–330 (2010).
Jiang, X. et al. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity 36, 959–973 (2012). References 57 and 58 reveal a crucial role of unanchored polyubiquitin chains in activating RIG-I and MDA5 signalling.
Wang, C. et al. The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 41, 919–933 (2014).
Shu, H. & Wang, Y. Adding to the STING. Immunity 41, 871–873 (2014).
Liu, J. et al. Rhomboid domain-containing protein 3 is a negative regulator of TLR3-triggered natural killer cell activation. Proc. Natl Acad. Sci. USA 110, 7814–7819 (2013).
Liu, J. et al. Rhbdd3 controls autoimmunity by suppressing the production of IL-6 by dendritic cells via K27-linked ubiquitination of the regulator NEMO. Nat. Immunol. 15, 612–622 (2014). This paper reports the key role of RHBDD3 in suppressing autoimmunity by controlling DC activation and the balance between T H 17 cells and regulatory T cells.
Levy, D. et al. Lysine methylation of the NF-κB subunit RelA by SETD6 couples activity of the histone methyltransferase GLP at chromatin to tonic repression of NF-κB signaling. Nat. Immunol. 12, 29–36 (2011). This paper shows the methylation of RELA by the lysine methyltransferase SETD6, which leads to suppression of RELA-induced inflammatory responses.
Ea, C. K. & Baltimore, D. Regulation of NF-κB activity through lysine monomethylation of p65. Proc. Natl Acad. Sci. USA 106, 18972–18977 (2009).
Lu, T. et al. Regulation of NF-κB by NSD1/FBXL11-dependent reversible lysine methylation of p65. Proc. Natl Acad. Sci. USA 107, 46–51 (2010).
Chi, H. & Flavell, R. A. Acetylation of MKP-1 and the control of inflammation. Sci. Signal. 1, e44 (2008).
Brestoff, J. R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 161, 146–160 (2015).
Pearce, E. J. & Everts, B. Dendritic cell metabolism. Nat. Rev. Immunol. 15, 18–29 (2015).
Everts, B. et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 15, 323–332 (2014).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013). This paper identifies a signalling role for succinate in innate immune responses by enhancing IL-1β production through HIF1α.
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Yan, Y. et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38, 1154–1163 (2013).
Williams-Bey, Y. et al. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS ONE 9, e97957 (2014).
Coulombe, F. et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity 40, 554–568 (2014).
Blanc, M. et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 38, 106–118 (2013).
Liu, S. Y. et al. Interferon-inducible cholesterol-25- hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 38, 92–105 (2013). References 75 and 76 demonstrate the antiviral activity of IFN-inducible product cholesterol-25-hydroxylase.
Reboldi, A. et al. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345, 679–684 (2014). This reference describes the inhibitory role of cholesterol-25-hydroxylase in NLRP3 inflammasome activation and IL-1β production.
Youm, Y. H. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 40, 616–619 (2010).
Chen, Z. J. Ubiquitin signalling in the NF-κB pathway. Nat. Cell Biol. 7, 758–765 (2005).
Wang, C. et al. The E3 ubiquitin ligase Nrdp1 'preferentially' promotes TLR-mediated production of type I interferon. Nat. Immunol. 10, 744–752 (2009).
Tanaka, T., Grusby, M. J. & Kaisho, T. PDLIM2-mediated termination of transcription factor NF-κB activation by intranuclear sequestration and degradation of the p65 subunit. Nat. Immunol. 8, 584–591 (2007).
Lin, A. E. et al. ARIH2 is essential for embryogenesis, and its hematopoietic deficiency causes lethal activation of the immune system. Nat. Immunol. 14, 27–33 (2013).
An, H. et al. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity 25, 919–928 (2006).
Hardin, A. O., Meals, E. A., Yi, T., Knapp, K. M. & English, B. K. SHP-1 inhibits LPS-mediated TNF and iNOS production in murine macrophages. Biochem. Biophys. Res. Commun. 342, 547–555 (2006).
Saitoh, T. et al. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolylisomerase Pin1. Nat. Immunol. 7, 598–605 (2006).
Kayagaki, N. et al. DUBA: a deubiquitinase that regulates type I interferon production. Science 318, 1628–1632 (2007).
An, H. et al. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat. Immunol. 9, 542–550 (2008).
Yang, K. et al. TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. J. Immunol. 182, 3782–3792 (2009).
Arimoto, K. et al. Polyubiquitin conjugation to NEMO by triparite motif protein 23 (TRIM23) is critical in antiviral defense. Proc. Natl Acad. Sci. USA 107, 15856–15861 (2010).
Yang, M. et al. E3 ubiquitin ligase CHIP facilitates Toll-like receptor signaling by recruiting and polyubiquitinating Src and atypical PKCζ. J. Exp. Med. 208, 2099–2112 (2011).
Piani, A. et al. Expression of MHC class II molecules contributes to lipopolysaccharide responsiveness. Eur. J. Immunol. 30, 3140–3146 (2000).
Liu, X. et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat. Immunol. 12, 416–424 (2011).
Liu, X. et al. Zinc finger protein ZBTB20 promotes Toll-like receptor-triggered innate immune responses by repressing IκBα gene transcription. Proc. Natl Acad. Sci. USA 110, 11097–11102 (2013).
Ozinsky, A. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl Acad. Sci. USA 97, 13766–13771 (2000).
Zhu, L. L. et al. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39, 324–334 (2013).
Napolitani, G., Rinaldi, A., Bertoni, F., Sallusto, F. & Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1- polarizing program in dendritic cells. Nat. Immunol. 6, 769–776 (2005). This study shows a synergistic interplay between TLRs to promote DCs that initiate T H 1 cell responses.
Sato, S. et al. Synergy and cross-tolerance between Toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165, 7096–7101 (2000).
Fritz, J. H. et al. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur. J. Immunol. 35, 2459–2470 (2005).
Tada, H., Aiba, S., Shibata, K., Ohteki, T. & Takada, H. Synergistic effect of Nod1 and Nod2 agonists with Toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect. Immun. 73, 7967–7976 (2005).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Bauernfeind, F. G. et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Franklin, B. S. et al. The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat. Immunol. 15, 727–737 (2014).
Baroja-Mazo, A. et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 15, 738–748 (2014).
Mayer-Barber, K. D. et al. Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184, 3326–3330 (2010).
Kanneganti, T. D. et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26, 433–443 (2007).
Rathinam, V. A. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150, 606–619 (2012).
Biswas, S. K. & Lopez-Collazo, E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 (2009).
Berghöfer, B., Haley, G., Frommer, T., Bein, G. & Hackstein, H. Natural and synthetic TLR7 ligands inhibit CpG-A− and CpG-C-oligodeoxynucleotide-induced IFN-α production. J. Immunol. 178, 4072–4079 (2007).
Marshall, J. D., Heeke, D. S., Gesner, M. L., Livingston, B. & Van Nest, G. Negative regulation of TLR9-mediated IFN-α induction by a small-molecule, synthetic TLR7 ligand. J. Leukoc. Biol. 82, 497–508 (2007).
Re, F. & Strominger, J. L. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J. Immunol. 173, 7548–7555 (2004).
Watanabe, T., Kitani, A., Murray, P. J. & Strober, W. NOD2 is a negative regulator of Toll-like receptor 2- mediated T helper type 1 responses. Nat. Immunol. 5, 800–808 (2004).
Watanabe, T. et al. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity 25, 473–485 (2006).
Maeda, S. et al. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1β processing. Science 307, 734–738 (2005). References 113 and 114 demonstrate the crosstalk between NOD2 signalling and TLR signalling and its role in the development of colitis.
Cui, J. et al. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat. Immunol. 13, 387–395 (2012).
Anand, P. K. et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012).
Allen, I. C. et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 36, 742–754 (2012).
Sun, K. & Metzger, D. W. Inhibition of pulmonary antibacterial defense by interferon-γ during recovery from influenza infection. Nat. Med. 14, 558–564 (2008).
Navarini, A. A. et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc. Natl Acad. Sci. USA 103, 15535–15539 (2006).
Negishi, H. et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat. Immunol. 13, 659–666 (2012). This study demonstrates that RLR-triggered IRF3 activation negatively modulates TLR responses by occupying the Il12b promoter over IRF5, and therefore provides a mechanistic explanation for the cross-interference of host defence during viral and bacterial co-infection.
Miettinen, M., Sareneva, T., Julkunen, I. & Matikainen, S. IFNs activate Toll-like receptor gene expression in viral infections. Genes Immun. 2, 349–355. (2001).
Lee, R. M., White, M. R. & Hartshorn, K. L. Influenza a viruses upregulate neutrophil Toll-like receptor 2 expression and function. Scand. J. Immunol. 63, 81–89 (2006).
Jamieson, A. M. et al. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science 340, 1230–1234 (2013).
Raby, A. C. et al. TLR activation enhances C5a-induced pro-inflammatory responses by negatively modulating the second C5a receptor, C5L2. Eur. J. Immunol. 41, 2741–2752 (2011).
Riedemann, N. C. et al. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB. J. 18, 370–372 (2004).
Zhang, X. et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 110, 228–236 (2007).
Mangsbo, S. M. et al. Complement activation by CpG in a human whole blood loop system: mechanisms and immunomodulatory effects. J. Immunol. 183, 6724–6732 (2009).
Zarewych, D. M., Kindzelskii, A. L., Todd, R. F. 3rd & Petty, H. R. LPS induces CD14 association with complement receptor type 3, which is reversed by neutrophil adhesion. J. Immunol. 156, 430–433 (1996).
Wang, M. et al. Microbial hijacking of complement-Toll-like receptor crosstalk. Sci. Signal. 3, ra11 (2010).
Hajishengallis, G. & Lambris, J. D. Microbial manipulation of receptor crosstalk in innate immunity. Nat. Rev. Immunol. 11, 187–200 (2011).
Schneider, M. et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 13, 823–831 (2012).
Allen, I. C. et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity 34, 854–865 (2011).
Xia, X. et al. NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34, 843–853 (2011).
Vande Walle, L. et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512, 69–73 (2014).
Onizawa, M. et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat. Immunol. 16, 618–627 (2015).
van Lent, P. L., Blom, A. B., Grevers, L., Sloetjes, A. & van den Berg, W. B. Toll-like receptor 4 induced FcγR expression potentiates early onset of joint inflammation and cartilage destruction during immune complex arthritis: Toll-like receptor 4 largely regulates FcγR expression by interleukin 10. Ann. Rheum. Dis. 66, 334–340 (2007).
Wenink, M. H. et al. The inhibitory FcγIIb receptor dampens TLR4-mediated immune responses and is selectively up-regulated on dendritic cells from rheumatoid arthritis patients with quiescent disease. J. Immunol. 183, 4509–4520 (2009).
Zhang, Y. et al. Immune complex/Ig negatively regulate TLR4-triggered inflammatory response in macrophages through FcγRIIb-dependent PGE2 production. J. Immunol. 182, 554–562 (2009).
Netea, M. G., Wijmenga, C. & O'Neill, L. A. Genetic variation in Toll-like receptors and disease susceptibility. Nat. Immunol. 13, 535–542 (2012).
Noguchi, E. et al. A Crohn's disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat. Immunol. 10, 471–479 (2009).
Cario, E., Gerken, G. & Podolsky, D. K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132, 1359–1374 (2007).
Solis, M., Goubau, D. & Hiscott, J. RIG-I has guts: identification of a role for RIG-I in colitis development. Cell Res. 17, 974–975 (2007).
van Heel, D. A. et al. Synergy between TLR9 and NOD2 innate immune responses is lost in genetic Crohn's disease. Gut 54, 1553–1557 (2005).
Kaczmarek, A., Brinkman, B. M., Heyndrickx, L., Vandenabeele, P. & Krysko, D. V. Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways. J. Pathol. 226, 598–608 (2012).
de Kivit, S., Tobin, M. C., Forsyth, C. B., Keshavarzian, A. & Landay, A. L. Regulation of intestinal immune responses through TLR activation: implications for pro- and prebiotics. Front. Immunol. 5, 60 (2014).
Cario, E. Barrier-protective function of intestinal epithelial Toll-like receptor 2. Mucosal Immunol. 1, S62–S66 (2008).
Isogawa, M., Robek, M. D., Furuichi, Y. & Chisari, F. V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 79, 7269–7272 (2005).
Chang, S., Dolganiuc, A. & Szabo, G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J. Leukoc. Biol. 82, 479–487 (2007).
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Saito, T. et al. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527 (2008).
Burdette, D. et al. Hepatitis C virus activates interleukin-1β via caspase-1-inflammasome complex. J. Gen. Virol. 93, 235–246 (2012).
Li, K. et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl Acad. Sci. USA 102, 2992–2997 (2005).
Abe, T. et al. Hepatitis C virus nonstructural protein 5A modulates the Toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J. Virol. 81, 8953–8966 (2007).
Otsuka, M. et al. Interaction between the HCV NS3 protein and the host TBK1 protein leads to inhibition of cellular antiviral responses. Hepatology 41, 1004–1012 (2005).
Sawhney, R. & Visvanathan, K. Polymorphisms of Toll-like receptors and their pathways in viral hepatitis. Antivir. Ther. 16, 443–458 (2011).
Szabo, G., Velayudham, A., Romics, L. J. & Mandrekar, P. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice:the role of Toll-like receptors 2 and 4. Alcohol. Clin. Exp. Res. 29, 140S–145S (2005).
Li, L. et al. Nuclear factor high-mobility group box 1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology 54, 1620–1630 (2011).
Rivera, C. A. et al. Toll-like receptor-4 signaling and Kupffer cells play pivotalroles in the pathogenesis of non-alcoholic steatohepatitis. J. Hepatol. 47, 571–579 (2007).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Yang, L. & Seki, E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front. Physiol. 3, 138 (2012).
Ouyang, X., Ghani, A. & Mehal, W. Z. Inflammasome biology in fibrogenesis. Biochim. Biophys. Acta. 1832, 979–988 (2013).
Hritz, I. et al. The critical role of Toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 48, 1224–1231 (2008).
Frantz, S., Ertl, G. & Bauersachs, J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 4, 444–454 (2007).
Hansson, G. K. & Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 (2011).
Monaco, C. et al. Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation 120, 2462–2469 (2009).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–13561 (2010).
Feng, Y. & Chao, W. Toll-like receptors and myocardial inflammation. Int. J. Inflam. 170352 (2011).
Buskiewicz, I. A. et al. c-FLIP-short reduces type I interferon production and increases viremia with coxsackievirus B3. PLoS ONE 9, e96156 (2014).
Hanke, M. L. & Kielian, T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin. Sci. 121, 367–387 (2011).
Furr, S. R., Chauhan, V. S., Sterka, D. Jr, Grdzelishvili, V. & Marriott, I. Characterization of retinoic acid-inducible gene-I expression in primary murine glia following exposure to vesicular stomatitis virus. J. Neurovirol. 14, 503–513 (2008).
Vezzani, A., Maroso, M., Balosso, S., Sanchez, M. A. & Bartfai, T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav. Immun. 25, 1281–1289 (2011).
Caso, J. R. et al. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 115, 1599–1608 (2007).
Pradillo, J. M. et al. Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J. Neurochem. 109, 287–294 (2009).
Fann, D. Y. et al. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res. Rev. 12, 941–966 (2013).
Minoretti, P. et al. Effect of the functional Toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer's disease. Neurosci. Lett. 391, 147–149 (2006).
Chen, Y. C. et al. Sequence variants of Toll like receptor 4 and late-onset Alzheimer's disease. PLoS ONE 7, e50771 (2012).
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 (2008).
Lee, Y. H., Lee, H. S., Choi, S. J., Ji, J. D. & Song, G. G. Associations between TLR polymorphisms and systemic lupus erythematosus: a systematic review and meta-analysis. Clin. Exp. Rheumatol. 30, 262–265 (2012).
Horton, C. G., Pan, Z. J. & Farris, A. D. Targeting Toll-like receptors for treatment of SLE. Mediators Inflamm. 2010, 498980 (2010).
Zhang, W. et al. AIM2 facilitates the apoptotic DNA-induced systemic lupus erythematosus via arbitrating macrophage functional maturation. J. Clin. Immunol. 33, 925–937 (2013).
Hernández-Pedro, N. Y., Espinosa-Ramirez, G., de la Cruz, V. P., Pineda, B. & Sotelo, J. Initial immunopathogenesis of multiple sclerosis: innate immune response. Clin. Dev. Immunol. 2013, 413465 (2013).
Touil, T., Fitzgerald, D., Zhang, G. X., Rostami, A. & Gran, B. TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-β. J. Immunol. 177, 7505–7509 (2006).
Liu, T., Gao, Y. J. & Ji, R. R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 28, 131–144 (2012).
Jin, C. & Flavell, R. A. Innate sensors of pathogen and stress: linking inflammation to obesity. J. Allergy Clin. Immunol. 132, 287–294 (2013).
Kopp, A. et al. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 17, 648–656 (2009).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Park, Y., Park, S., Yoo, E., Kim, D. & Shin, H. Association of the polymorphism for Toll-like receptor 2 with type 1 diabetes susceptibility. Ann. NY Acad. Sci. 1037, 170–174 (2004).
Pirie, F. J. et al. Toll-like receptor 3 gene polymorphisms in South African Blacks with type 1 diabetes. Tissue Antigens 66, 125–130 (2005).
Zipris, D. Toll-like receptors and type 1 diabetes. Adv. Exp. Med. Biol. 654, 585–610 (2010).
Muoio, D. M. & Newgard, C. B. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell. Biol. 9, 193–205 (2008).
Jourdan, T. et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates β-cell loss in type 2 diabetes. Nat. Med. 19, 1132–1140 (2013).
Acknowledgements
This work was supported by the National Key Basic Research Program of China (2013CB530503) and the National Natural Science Foundation of China (31390431, 81123006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- E3 ubiquitin ligase
-
An enzyme that is required to attach the molecular tag ubiquitin to proteins. Depending on the position and number of ubiquitin molecules that are attached, the ubiquitin tag can target proteins for degradation in the proteasomal complex, create a scaffold for assembly of signalling complexes, sort them to specific subcellular compartments or modify their biological activity.
- Epigenetic modifications
-
Hereditable regulation of genes achieved without altering the genome sequence, such as through DNA methylation, histone modifications and non-coding RNAs.
- DNA methyltransferases
-
(DNMTs). Enzymes that transfer methyl groups from S-adenosylmethionine to specific adenines or cytosines in genomic DNA. DNA methylation is crucial for the regulation of chromatin remodelling and gene expression.
- M2 macrophage
-
A macrophage that is stimulated by interleukin-4 (IL-4) or IL-13 and that expresses arginase 1, the mannose receptor CD206 and the IL-4 receptor α-chain.
- Long non-coding RNAs
-
(lncRNAs). Non-coding RNAs that are transcribed by RNA polymerase II, have more than 200 nucleotides and have no ability to code protein.
- Succinate
-
Also known as butanedioic acid. The key intermediate in the tricarboxylic acid cycle and also one of the fermentation products of anaerobic metabolism.
- Prostaglandin
-
Cyclopentane ring-containing lipid derived from the metabolism of arachidonic acid by cyclooxygenases and downstream synthase enzymes. Prostaglandins have a diverse range of biological activities and a well-recognized role in inflammation and pain.
- Ketone bodies
-
The three endogenous molecules (acetone, acetoacetic acid and β-hydroxybutyric acid) that are produced from fatty acids in the liver during periods of starvation or carbohydrate restriction, when they serve as energy sources for the body.
- Complement system
-
Three independent pathways can lead to activation of the complement cascade. The classical pathway is activated via C1q binding to immune complexes, the alternative pathway is triggered by direct C3 activation, and the lectin pathway is initiated by the binding of mannose-binding lectin (MBL) to the surface of microorganisms and other activating surfaces.
- Pyroptosis
-
A type of programmed cell death that features rapid rupture of the plasma membrane, depends on caspase 1 activation and is associated with the release of inflammatory cytokines and induction of the host antimicrobial inflammatory response.
- Necroptosis
-
A pro-inflammatory form of caspase-independent cell death. Necroptotic cells display swelling of cellular organelles, cell membrane rupturing and uncontrolled release of cellular contents into the surrounding tissue, ultimately followed by cell death.
Rights and permissions
About this article
Cite this article
Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol 16, 35–50 (2016). https://doi.org/10.1038/nri.2015.8
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2015.8
This article is cited by
-
Sterile inflammation via TRPM8 RNA-dependent TLR3-NF-kB/IRF3 activation promotes antitumor immunity in prostate cancer
The EMBO Journal (2024)
-
Emerging role of microbiota derived outer membrane vesicles to preventive, therapeutic and diagnostic proposes
Infectious Agents and Cancer (2023)
-
RBP–RNA interactions in the control of autoimmunity and autoinflammation
Cell Research (2023)
-
Lysine methyltransferase SMYD2 inhibits antiviral innate immunity by promoting IRF3 dephosphorylation
Cell Death & Disease (2023)
-
WDR77 inhibits prion-like aggregation of MAVS to limit antiviral innate immune response
Nature Communications (2023)