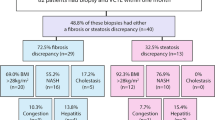
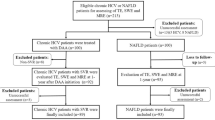
Key Points
-
NAFLD is the most common form of chronic liver disease, and patient-centred risk-assessment strategies are therefore needed for cost-effective care
-
Liver elastography — or liver stiffness measurement — is an alternative to liver biopsy to evaluate patients with NAFLD for the presence of advanced fibrosis or cirrhosis
-
Of the available elastographic modalities, vibration-controlled transient elastography is the most studied and magnetic resonance elastography is the most accurate; ultrasound-based elastography is promising but lacks defined examination quality criteria
-
Future research is needed to establish the optimal sequence of modalities for use in the clinic and the definition of clinically meaningful changes in liver stiffness
Abstract
NAFLD is a global epidemic. The prevalence of NAFLD is 20–30% in North America, northern Europe, Australia, Japan, India and China. It is crucial that patients with NAFLD receive an assessment for their risk of advanced fibrosis, which increases the risk of hepatocellular carcinoma and other complications of cirrhosis. Risk stratification that is efficient, cost-effective, patient-centred and evidence-based is one of the most important issues facing clinicians who care for those with liver disease. Given patients' preference to avoid liver biopsy, noninvasive alternatives to assess liver fibrosis are in high demand. The most accurate noninvasive methods are based on liver elastography. Research on these techniques — which include vibration-controlled transient elastography (VCTE), magnetic resonance elastography (MRE), shear-wave elastography and acoustic radiation force impulse — has proliferated. Unfortunately, the literature has not kept pace with clinical practice. There is limited guidance for how clinicians should anticipate and manage the pitfalls of these tests. Furthermore, guidance is unavailable for clinicians regarding the optimal incorporation of VCTE, MRE or the emerging elastographic techniques into their clinical strategy, particularly for patients with NAFLD. In this Review, we summarize the available evidence, highlight gaps to address in further research and explore optimization of these techniques in clinical practice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vernon, G., Baranova, A. & Younossi, Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 34, 274–285 (2011).
Williams, C. D. et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140, 124–131 (2011).
Mozumdar, A. & Liguori, G. Persistent increase of prevalence of metabolic syndrome among US adults: NHANES III to NHANES 1999–2006. Diabetes Care 34, 216–219 (2011).
Peery, A. F. et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143, 1179–1187.e3 (2012).
Ekstedt, M. et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554 (2015).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397.e10 (2012).
Pasha, T., Gabriel, S., Therneau, T., Dickson, E. R. & Lindor, K. D. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 27, 1220–1226 (1998).
Rockey, D. C., Caldwell, S. H., Goodman, Z. D., Nelson, R. C. & Smith, A. D. Liver biopsy. Hepatology 49, 1017–1044 (2009).
Foster, G. et al. Management of chronic hepatitis C: clinical audit of biopsy based management algorithm. BMJ 315, 453–458 (1997).
Bedossa, P., Dargère, D. & Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38, 1449–1457 (2003).
Bedossa, P. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 20, 15–20 (1994).
Tapper E. B. & Lok A. S. F. Use of liver imaging and biopsy in clinical practice. N. Engl. J. Med. 377, 756–768 (2017).
Dietrich, C. F. et al. EFSUMB Guidelines and Recommendations on the clinical use of liver ultrasound elastography, update 2017 (Long version). Ultraschall Med. 34, 169–184 (2013).
Tapper, E. B. et al. Levels of alanine aminotransferase confound use of transient elastography to diagnose fibrosis in patients with chronic hepatitis C virus infection. Clin. Gastroenterol. Hepatol. 10, 932–937.e1 (2012).
Tapper, E. B., Castera, L. & Afdhal, N. H. FibroScan (vibration-controlled transient elastography): where does it stand in the United States practice. Clin. Gastroenterol. Hepatol. 13, 27–36 (2015).
Boursier, J. et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J. Hepatol. 65, 570–578 (2016).
Castéra, L. et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 51, 828–835 (2010).
Park, C. C. et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 152, 598–607.e2 (2017). This study performs a head to head comparison of VCTE and MRE in a US cohort.
Cassinotto, C. et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 63, 1817–1827 (2016). This is the only study to evaluate VCTE, SWE and ARFI in patients with NAFLD.
Tapper, E. B., Challies, T., Nasser, I., Afdhal, N. H. & Lai, M. The performance of vibration controlled transient elastography in a us cohort of patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 111, 677–684 (2016). The first report of VCTE use for NAFLD in the US (using the M probe).
Wong V. W. et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 107, 1862–1871 (2012).
Imajo, K. et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 150, 626–637.e7 (2016).
Chen, J. et al. Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology 283, 418–428 (2017).
Karlas, T. et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 66, 1022–1030 (2017).
Caussy, C. et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology https://doi.org/10.1002/hep.29639 (2017).
Wong V. W. et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J. Hepatol. 67, 577–584 (2017).
Petta, S. et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology 65, 1145–1155 (2017). This study uses CAP during VCTE exams to account for the contribution of steatosis to liver stiffness.
Deffieux, T. et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J. Hepatol. 62, 317–324 (2015).
Cassinotto, C. et al. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J. Hepatol. 61, 550–557 (2014).
Friedrich-Rust, M. et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 252, 595–604 (2009).
Cui, J. et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 63, 453–461 (2016).
Palmeri, M. L. et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J. Hepatol. 55, 666–672 (2011).
Ebinuma, H. et al. Evaluation of liver fibrosis by transient elastography using acoustic radiation force impulse: comparison with Fibroscan®. J. Gastroenterol. 46, 1238 (2011).
Yoon, K. T. et al. Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig. Dis. Sci. 57, 1682–1691 (2012).
Venkatesh, S. K., Yin, M. & Ehman, R. L. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J. Magnet. Resonance Imag. 37, 544–555 (2013).
Loomba, R. et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. Am. J. Gastroenterol. 111, 986–994 (2016).
Loomba, R. et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 60, 1920–1928 (2014).
Wagner, M. et al. Technical failure of MR elastography examinations of the liver: experience from a large single-center study. Radiology 284, 401–412 (2017).
Cui, J. et al. Comparative diagnostic accuracy of magnetic resonance elastography versus eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment. Pharmacol. Ther. 41, 1271–1280 (2015).
Dulai, P. S., Sirlin, C. B. & Loomba, R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J. Hepatol. 65, 1006–1016 (2016).
Cui, J. et al. Sitagliptin versus placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J. Hepatol. 65, 369–376 (2016).
Loomba, R. et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 61, 1239–1250 (2015).
Noureddin, M. et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 58, 1930–1940 (2013).
Le, T. A. et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 56, 922–932 (2012).
Satkunasingham, J. et al. Can negligible hepatic steatosis determined by MRI-proton density fat fraction obviate the need for liver biopsy in potential liver donors? Liver Transpl. https://doi.org/10.1002/lt.24965 (2017).
Lin, S. C. et al. Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin. Gastroenterol. Hepatol. 13, 1337–1345.e6 (2015).
Tapper, E. B., Sengupta, N., Hunink, M. M., Afdhal, N. H. & Lai, M. Cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration controlled transient elastography. Am. J. Gastroenterol. 110, 1298–1304 (2015).
Bruix, J. & Sherman, M. Management of hepatocellular carcinoma: an update. Hepatology 53, 1020–1022 (2011).
Garcia-Tsao, G., Abraldes, J. G., Berzigotti, A. & Bosch, J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 65, 310–335 (2017).
Sanyal, A. J. et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases–US Food and Drug Administration Joint Workshop. Hepatology 61, 1392–1405 (2015).
Patel, N. S. et al. Weight loss decreases magnetic resonance elastography estimated liver stiffness in nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 15, 463–464 (2017).
Tapper, E. B., Hunink, M. M., Afdhal, N. H., Lai, M. & Sengupta, N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the Primary Care Physician using the NAFLD fibrosis score. PLOS ONE 11, e0147237 (2016).
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55, 2005–2023 (2012).
Petta, S. et al. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology 62, 1101–1110 (2015).
Kumar, R. et al. Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: diagnostic performance and clinicopathological correlation. Dig. Dis. Sci. 58, 265–274 (2013).
Chan, W.-K., Mustapha, N. R. N. & Mahadeva, S. A novel 2-step approach combining the NAFLD fibrosis score and liver stiffness measurement for predicting advanced fibrosis. Hepatol. Int. 9, 594–602 (2015).
Aykut, U. E. et al. A comparison of FibroMeter NAFLD Score, NAFLD fibrosis score, and transient elastography as noninvasive diagnostic tools for hepatic fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 49, 1343–1348 (2014).
Naveau, S. et al. The diagnostic accuracy of transient elastography for the diagnosis of liver fibrosis in bariatric surgery candidates with suspected NAFLD. Obes. Surg. 24, 1693–1701 (2014).
Gaia, S. et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J. Hepatol. 54, 64–71 (2011).
Mahadeva, S. et al. Performance of transient elastography (TE) and factors associated with discordance in non-alcoholic fatty liver disease. J. Digestive Diseases 14, 604–610 (2013).
Wong, V. W. S. et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 51, 454–462 (2010).
Ochi, H. et al. Real-time tissue elastography for evaluation of hepatic fibrosis and portal hypertension in nonalcoholic fatty liver diseases. Hepatology 56, 1271–1278 (2012).
Acknowledgements
R.L. is supported in part by the grant R01-DK106419-03. Research reported in this publication was supported in part by the US National Institute of Environmental Health Sciences of the US National Institutes of Health under award number P42ES010337. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US NIH.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to this article.
Corresponding author
Ethics declarations
Competing interests
R.L. has received research funding support from General Electric and Siemens.
Rights and permissions
About this article
Cite this article
Tapper, E., Loomba, R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol 15, 274–282 (2018). https://doi.org/10.1038/nrgastro.2018.10
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2018.10
This article is cited by
-
Tethered spinal cord tension assessed via ultrasound elastography in computational and intraoperative human studies
Communications Medicine (2024)
-
Role of Vibration-Controlled Transient Elastography in the Evaluation and Management of Metabolic Dysfunction-Associated Steatotic Liver Disease
Current Hepatology Reports (2024)
-
Association of weight-adjusted-waist index with non-alcoholic fatty liver disease and liver fibrosis: a cross-sectional study based on NHANES
European Journal of Medical Research (2023)
-
Magnetic resonance elastography-derived stiffness: potential imaging biomarker for differentiation of benign and malignant pancreatic masses
Abdominal Radiology (2023)
-
Global, regional, and national burden of liver cancer due to non-alcoholic steatohepatitis, 1990–2019: a decomposition and age–period–cohort analysis
Journal of Gastroenterology (2023)