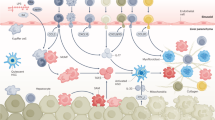
Key Points
-
Activation of hepatic stellate cells (HSCs) into proliferative, fibrogenic myofibroblasts is well established as the central driver of hepatic fibrosis in experimental and human liver injury
-
A panoply of intracellular events and signals in all cellular compartments drive the activated phenotype of HSCs, and many of these represent potential targets for antifibrotic therapies
-
Extracellular signals converging upon HSCs to promote their activation include those originating from the extracellular matrix and stimuli from resident and infiltrating inflammatory cells
-
Emerging concepts in HSC activation focus on novel mediators and intracellular signals, as well as drivers of HSC inactivation, which collectively have generated a template for uncovering novel therapeutic targets
Abstract
Hepatic fibrosis is a dynamic process characterized by the net accumulation of extracellular matrix resulting from chronic liver injury of any aetiology, including viral infection, alcoholic liver disease and NASH. Activation of hepatic stellate cells (HSCs) — transdifferentiation of quiescent, vitamin-A-storing cells into proliferative, fibrogenic myofibroblasts — is now well established as a central driver of fibrosis in experimental and human liver injury. Yet, the continued discovery of novel pathways and mediators, including autophagy, endoplasmic reticulum stress, oxidative stress, retinol and cholesterol metabolism, epigenetics and receptor-mediated signals, reveals the complexity of HSC activation. Extracellular signals from resident and inflammatory cells including macrophages, hepatocytes, liver sinusoidal endothelial cells, natural killer cells, natural killer T cells, platelets and B cells further modulate HSC activation. Finally, pathways of HSC clearance have been greatly clarified, and include apoptosis, senescence and reversion to an inactivated state. Collectively, these findings reinforce the remarkable complexity and plasticity of HSC activation, and underscore the value of clarifying its regulation in hopes of advancing the development of novel diagnostics and therapies for liver disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Trautwein, C., Friedman, S. L., Schuppan, D. & Pinzani, M. Hepatic fibrosis: concept to treatment. J. Hepatol. 62, S15–S24 (2015).
Lee, Y. A., Wallace, M. C. & Friedman, S. L. Pathobiology of liver fibrosis: a translational success story. Gut 64, 830–841 (2015).
Puche, J. E., Saiman, Y. & Friedman, S. L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 3, 1473–1492 (2013).
Ellis, E. L. & Mann, D. A. Clinical evidence for the regression of liver fibrosis. J. Hepatol. 56, 1171–1180 (2012).
Marcellin, P. et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 381, 468–475 (2013).
Chang, T. T. et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 52, 886–893 (2010).
D'Ambrosio, R. et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 56, 532–543 (2012).
Wake, K. “Sternzellen” in the liver: perisinuosoidal cells with special reference to storage of vitamin A. Am. J. Anat. 132, 429–462 (1971).
Friedman, S. L. & Roll, F. J. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal. Biochem. 161, 207–218 (1987).
Mederacke, I., Dapito, D. H., Affo, S., Uchinami, H. & Schwabe, R. F. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat. Protoc. 10, 305–315 (2015).
Mederacke, I. et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 4, 2823 (2013).
Iwaisako, K. et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc. Natl Acad. Sci. USA 111, E3297–E3305 (2014).
Lemoinne, S., Cadoret, A., Mourabit, H. E., Thabut, D. & Housset, C. Origins and functions of liver myofibroblasts. Biochim. Biophys. Acta 1832, 948–954 (2013).
Kluwe, J. et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut 60, 1260–1268 (2011).
Henderson, N. C. et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19, 1617–1624 (2013).
Yin, C., Evason, K. J., Maher, J. J. & Stainier, D. Y. The basic helix-loop-helix transcription factor, heart and neural crest derivatives expressed transcript 2, marks hepatic stellate cells in zebrafish: analysis of stellate cell entry into the developing liver. Hepatology 56, 1958–1970 (2012).
Geerts, A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 21, 311–335 (2001).
Miyata, E. et al. Hematopoietic origin of hepatic stellate cells in the adult liver. Blood 111, 2427–2435 (2008).
Zhang, D. Y. et al. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut 65, 1754–1764 (2016).
Friedman, S. L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 275, 2247–2250 (2000).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Schnabl, B., Choi, Y. H., Olsen, J. C., Hagedorn, C. H. & Brenner, D. A. Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab. Invest. 82, 323–333 (2002).
Iredale, J. P. et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549 (1998).
Kisseleva, T. et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl Acad. Sci. USA 109, 9448–9453 (2012).
Troeger, J. S. et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 143, 1073–1083.e22 (2012).
Mehal, W. Z., Iredale, J. & Friedman, S. L. Scraping fibrosis: expressway to the core of fibrosis. Nat. Med. 17, 552–553 (2011).
Hellerbrand, C., Stefanovic, B., Giordano, F., Burchardt, E. R. & Brenner, D. A. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo . J. Hepatol. 30, 77–87 (1999).
Breitkopf, K., Godoy, P., Ciuclan, L., Singer, M. V. & Dooley, S. TGF-beta/Smad signaling in the injured liver. Z. Gastroenterol. 44, 57–66 (2006).
Friedman, S. L. Hepatic stellate cells — protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172 (2008).
Hanafusa, H. et al. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J. Biol. Chem. 274, 27161–27167 (1999).
Engel, M. E., McDonnell, M. A., Law, B. K. & Moses, H. L. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J. Biol. Chem. 274, 37413–37420 (1999).
Wong, L., Yamasaki, G., Johnson, R. J. & Friedman, S. L. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J. Clin. Invest. 94, 1563–1569 (1994).
Pinzani, M. PDGF and signal transduction in hepatic stellate cells. Front. Biosci. 7, d1720–d1726 (2002).
Kocabayoglu, P. et al. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J. Hepatol. 63, 141–147 (2015).
Yang, L. et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology 146, 1339–1350.e1 (2014).
Kantari-Mimoun, C. et al. Resolution of liver fibrosis requires myeloid cell-driven sinusoidal angiogenesis. Hepatology 61, 2042–2055 (2015).
Huang, G. & Brigstock, D. R. Regulation of hepatic stellate cells by connective tissue growth factor. Front. Biosci. 17, 2495–2507 (2015).
Chen, L. et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 59, 1118–1129 (2014).
Chen, L., Chen, R., Kemper, S., Charrier, A. & Brigstock, D. R. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: role of exosomes in horizontal transfer of Twist1. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G491–G499 (2015).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT01217632 (2016).
Olaso, E. et al. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J. Clin. Invest. 108, 1369–1378 (2001).
Schuppan, D., Ruehl, M., Somasundaram, R. & Hahn, E. G. Matrix as a modulator of hepatic fibrogenesis. Semin. Liver Dis. 21, 351–372 (2001).
Martin, K. et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat. Commun. 7, 12502 (2016).
Ikeda, K. et al. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J. Biol. Chem. 277, 19206–19212 (2002).
Omenetti, A., Choi, S., Michelotti, G. & Diehl, A. M. Hedgehog signaling in the liver. J. Hepatol. 54, 366–373 (2011).
Michelotti, G. A. et al. Smoothened is a master regulator of adult liver repair. J. Clin. Invest. 123, 2380–2394 (2013).
Syn, W. K. et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology 53, 106–115 (2012).
Urtasun, R. et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology 55, 594–608 (2012).
Machado, M. V. et al. Reduced lipoapoptosis, hedgehog pathway activation and fibrosis in caspase-2 deficient mice with non-alcoholic steatohepatitis. Gut 64, 1148–1157 (2015).
Greenbaum, L. E. & Wells, R. G. The role of stem cells in liver repair and fibrosis. Int. J. Biochem. Cell Biol. 43, 222–229 (2011).
Swiderska-Syn, M. et al. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut 63, 1333–1344 (2014).
Xie, G. et al. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology 58, 1801–1813 (2013).
Hernandez-Gea, V. et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142, 938–946 (2012).
Thoen, L. F. et al. A role for autophagy during hepatic stellate cell activation. J. Hepatol. 55, 1353–1360 (2011).
Hernandez-Gea, V. et al. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J. Hepatol. 59, 98–104 (2013).
Koo, J. H., Lee, H. J., Kim, W. & Kim, S. G. Endoplasmic reticulum stress in hepatic stellate cells promotes liver fibrosis via PERK-mediated degradation of HNRNPA1 and up-regulation of SMAD2. Gastroenterology 150, 181–193.e8 (2016).
Kim, R. S. et al. The XBP1 arm of the unfolded protein response induces fibrogenic activity in hepatic stellate cells through autophagy. Sci. Rep. 6, 39342 (2016).
Sato, Y. et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 26, 431–442 (2008).
Kawasaki, K. et al. Deletion of the collagen-specific molecular chaperone Hsp47 causes endoplasmic reticulum stress-mediated apoptosis of hepatic stellate cells. J. Biol. Chem. 290, 3639–3646 (2015).
Seki, E., Brenner, D. A. & Karin, M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 143, 307–320 (2012).
Kluwe, J. et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology 138, 347–359 (2010).
Zhao, G. et al. Jnk1 in murine hepatic stellate cells is a crucial mediator of liver fibrogenesis. Gut 63, 1159–1172 (2014).
Novo, E. et al. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells. J. Hepatol. 54, 964–974 (2011).
Novo, E. & Parola, M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair 1, 5 (2008).
Lan, T., Kisseleva, T. & Brenner, D. A. Deficiency of NOX1 or NOX4 prevents liver inflammation and fibrosis in mice through inhibition of hepatic stellate cell activation. PLoS ONE 10, e0129743 (2015).
Jiang, J. X. et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo . Gastroenterology 139, 1375–1384 (2010).
Aoyama, T. et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56, 2316–2327 (2012).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT02010242 (2015).
Tomita, K. et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology 59, 154–169 (2014).
Yi, H. S. et al. Alcohol dehydrogenase III exacerbates liver fibrosis by enhancing stellate cell activation and suppressing natural killer cells in mice. Hepatology 60, 1044–1053 (2014).
Valenti, L. et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 51, 1209–1217 (2010).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008).
Pirazzi, C. et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum. Mol. Genet. 23, 4077–4085 (2014).
Teratani, T. et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology 142, 152–164.e10 (2012).
Tomita, K. et al. Acyl-CoA:cholesterol acyltransferase 1 mediates liver fibrosis by regulating free cholesterol accumulation in hepatic stellate cells. J. Hepatol. 61, 98–106 (2014).
Van Rooyen, D. M. et al. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J. Hepatol. 59, 144–152 (2013).
Saxena, N. K. & Anania, F. A. Adipocytokines and hepatic fibrosis. Trends Endocrinol. Metab. 26, 153–161 (2015).
Shafiei, M. S., Shetty, S., Scherer, P. E. & Rockey, D. C. Adiponectin regulation of stellate cell activation via PPARgamma-dependent and -independent mechanisms. Am. J. Pathol. 178, 2690–2699 (2011).
Kamada, Y. et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology 125, 1796–1807 (2003).
Coombes, J. D. et al. Osteopontin is a proximal effector of leptin-mediated non-alcoholic steatohepatitis (NASH) fibrosis. Biochim. Biophys. Acta 1862, 135–144 (2016).
Sahai, A. et al. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1035–G1043 (2004).
Coll, M. et al. Integrative miRNA and gene expression profiling analysis of human quiescent hepatic stellate cells. Sci. Rep. 5, 11549 (2015).
Zhang, Z. et al. The auto-regulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (miR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J. Biol. Chem. http://dx.doi.org/10.1074/jbc.M113.517953 (2013).
Ogawa, T. et al. MicroRNA-221/222 upregulation indicates the activation of stellate cells and the progression of liver fibrosis. Gut 61, 1600–1609 (2012).
Ji, J. et al. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 583, 759–766 (2009).
Hyun, J. et al. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat. Commun. 7, 10993 (2016).
Liu, X. Y. et al. Induction of autophagy and apoptosis by miR-148a through the sonic hedgehog signaling pathway in hepatic stellate cells. Am. J. Cancer Res. 5, 2569–2589 (2015).
Jung, K. H. et al. Differentiation therapy for hepatocellular carcinoma: multifaceted effects of miR-148a on tumor growth and phenotype and liver fibrosis. Hepatology 63, 864–879 (2016).
Huang, Y. H. et al. Activation of Mir-29a in activated hepatic stellate cells modulates its profibrogenic phenotype through inhibition of histone deacetylases 4. PLoS ONE 10, e0136453 (2015).
Guo, C. J., Pan, Q., Li, D. G., Sun, H. & Liu, B. W. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: an essential role for apoptosis. J. Hepatol. 50, 766–778 (2009).
Li, J. et al. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J. Hepatol. 58, 522–528 (2013).
Roderburg, C. et al. miR-133a mediates TGF-beta-dependent derepression of collagen synthesis in hepatic stellate cells during liver fibrosis. J. Hepatol. 58, 736–742 (2013).
Sekiya, Y. et al. Down-regulation of cyclin E1 expression by microRNA-195 accounts for interferon-beta-induced inhibition of hepatic stellate cell proliferation. J. Cell. Physiol. 226, 2535–2542 (2011).
Venugopal, S. K. et al. Liver fibrosis causes down-regulation of miRNA-150 and miRNA-194 in hepatic stellate cells and their over-expression causes decreased stellate cell activation. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G101–G106 (2009).
Zhou, C. et al. Long noncoding RNAs expressed in human hepatic stellate cells form networks with extracellular matrix proteins. Genome Med. 8, 31 (2016).
Tian, W. et al. Myocardin-related transcription factor A (MRTF-A) plays an essential role in hepatic stellate cell activation by epigenetically modulating TGF-beta signaling. Int. J. Biochem. Cell Biol. 71, 35–43 (2016).
Hardy, T. & Mann, D. A. Epigenetics in liver disease: from biology to therapeutics. Gut 65, 1895–1905 (2016).
Bian, E. B. et al. DNMT1-mediated PTEN hypermethylation confers hepatic stellate cell activation and liver fibrogenesis in rats. Toxicol. Appl. Pharmacol. 264, 13–22 (2012).
Mann, J. et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology 138, 705–714 (2010).
Kweon, S. M., Chi, F., Higashiyama, R., Lai, K. & Tsukamoto, H. Wnt pathway stabilizes MeCP2 protein to repress PPAR-gamma in Activation of hepatic stellate cells. PLoS ONE 11, e0156111 (2016).
Seki, E. et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Gabele, E. et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem. Biophys. Res. Commun. 376, 271–276 (2008).
Miura, K. et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 57, 577–589 (2013).
Byun, J. S., Suh, Y. G., Yi, H. S., Lee, Y. S. & Jeong, W. I. Activation of Toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J. Hepatol. 58, 342–349 (2013).
Chou, M. H. et al. Selective activation of Toll-like receptor 7 in activated hepatic stellate cells may modulate their profibrogenic phenotype. Biochem. J. 447, 25–34 (2012).
Huang, H. et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology 46, 297–306 (2007).
Guo, J. et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology 49, 960–968 (2009).
Seo, W. et al. Exosome-mediated activation of Toll-like receptor 3 in stellate cells stimulates interleukin-17 production by gammadelta T cells in liver fibrosis. Hepatology 64, 616–631 (2016).
Pinzani, M. & Marra, F. Cytokine receptors and signaling in hepatic stellate cells. Semin. Liver Dis. 21, 397–416 (2001).
Jiao, J. et al. Interleukin-15 receptor alpha on hepatic stellate cells regulates hepatic fibrogenesis in mice. J. Hepatol. 65, 344–353 (2016).
Meng, F. et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143, 765–776.e3 (2012).
Chiu, Y. S., Wei, C. C., Lin, Y. J., Hsu, Y. H. & Chang, M. S. IL-20 and IL-20R1 antibodies protect against liver fibrosis. Hepatology 60, 1003–1014 (2014).
Kong, X. et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 56, 1150–1159 (2012).
Beaven, S. W. et al. Liver x receptor signaling is a determinant of stellate cell activation and susceptibility to fibrotic liver disease. Gastroenterology 140, 1052–1062 (2011).
O'Mahony, F. et al. Liver X receptors balance lipid stores in hepatic stellate cells through Rab18, a retinoid responsive lipid droplet protein. Hepatology 62, 615–626 (2015).
Kong, B., Luyendyk, J. P., Tawfik, O. & Guo, G. L. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 328, 116–122 (2009).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT02548351 (2017).
Hazra, S. et al. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J. Biol. Chem. 279, 11392–11401 (2004).
Moran-Salvador, E. et al. Cell-specific PPARgamma deficiency establishes anti-inflammatory and anti-fibrogenic properties for this nuclear receptor in non-parenchymal liver cells. J. Hepatol. 59, 1045–1053 (2013).
Iwaisako, K. et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor delta agonist. Proc. Natl Acad. Sci. USA 109, E1369–E1376 (2012).
Staels, B. et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 58, 1941–1952 (2013).
Ratziu, V. et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 150, 1147–1159.e5 (2016).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT02704403 (2017).
Ding, N. et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 153, 601–613 (2013).
Duran, A. et al. p62/SQSTM1 by binding to vitamin d receptor inhibits hepatic stellate cell activity, fibrosis, and liver cancer. Cancer Cell 30, 595–609 (2016).
Li, T. et al. Novel role of nuclear receptor Rev-erbα in hepatic stellate cell activation: potential therapeutic target for liver injury. Hepatology 59, 2383–2396 (2014).
Palumbo-Zerr, K. et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat. Med. 21, 150–158 (2015).
Teixeira-Clerc, F. et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat. Med. 12, 671–676 (2006).
Julien, B. et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 128, 742–755 (2005).
Munoz-Luque, J. et al. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J. Pharmacol. Exp. Ther. 324, 475–483 (2008).
Guillot, A. et al. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology 59, 296–306 (2014).
Granzow, M. et al. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology 60, 334–348 (2014).
Knight, V., Tchongue, J., Lourensz, D., Tipping, P. & Sievert, W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology 55, 879–887 (2012).
Seki, E. et al. CCR2 promotes hepatic fibrosis in mice. Hepatology 50, 185–197 (2009).
Lefebvre, E. et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS ONE 11, e0158156 (2016).
Ruddell, R. G. et al. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am. J. Pathol. 169, 861–876 (2006).
Kim, D. C. et al. 5-HT2A receptor antagonists inhibit hepatic stellate cell activation and facilitate apoptosis. Liver Int. 33, 535–543 (2013).
Ebrahimkhani, M. R. et al. Stimulating healthy tissue regeneration by targeting the 5-HT(2B) receptor in chronic liver disease. Nat. Med. 17, 1668–1673 (2011).
Li, Y. H., Woo, S. H., Choi, D. H. & Cho, E. H. Succinate causes alpha-SMA production through GPR91 activation in hepatic stellate cells. Biochem. Biophys. Res. Commun. 463, 853–858 (2015).
Li, Y. H. et al. Sirtuin 3 (SIRT3) regulates alpha-smooth muscle actin (alpha-SMA) production through the succinate dehydrogenase-G protein-coupled receptor 91 (GPR91) pathway in hepatic stellate cells. J. Biol. Chem. 291, 10277–10292 (2016).
Lopez-Sanchez, I. et al. GIV/Girdin is a central hub for profibrogenic signalling networks during liver fibrosis. Nat. Commun. 5, 4451 (2014).
Aylon, Y. et al. The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. 30, 786–797 (2016).
Halder, G. & Johnson, R. L. Hippo signaling: growth control and beyond. Development 138, 9–22 (2011).
Moroishi, T., Hansen, C. G. & Guan, K. L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 15, 73–79 (2015).
Yu, F. X. & Guan, K. L. The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 (2013).
Mannaerts, I. et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol. 63, 679–688 (2015).
Swiderska-Syn, M. et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology 64, 232–244 (2016).
Machado, M. V. et al. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. J. Hepatol. 63, 962–970 (2015).
Wilhelm, A. et al. CD248/endosialin critically regulates hepatic stellate cell proliferation during chronic liver injury via a PDGF-regulated mechanism. Gut 65, 1175–1185 (2016).
Mogler, C. et al. Hepatic stellate cell-expressed endosialin balances fibrogenesis and hepatocyte proliferation during liver damage. EMBO Mol. Med. 7, 332–338 (2015).
Ding, N. et al. BRD4 is a novel therapeutic target for liver fibrosis. Proc. Natl Acad. Sci. USA 112, 15713–15718 (2015).
Henderson, N. C. et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl Acad. Sci. USA 103, 5060–5065 (2006).
Traber, P. G. et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE 8, e75361 (2013).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT02462967 (2017).
Delgado, I. et al. GATA4 loss in the septum transversum mesenchyme promotes liver fibrosis in mice. Hepatology 59, 2358–2370 (2014).
Wandzioch, E., Kolterud, A., Jacobsson, M., Friedman, S. L. & Carlsson, L. Lhx2−/− mice develop liver fibrosis. Proc. Natl Acad. Sci. USA 101, 16549–16554 (2004).
Arndt, S. et al. Enhanced expression of BMP6 inhibits hepatic fibrosis in non-alcoholic fatty liver disease. Gut 64, 973–981 (2015).
Barcena, C. et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J. Hepatol. 63, 670–678 (2015).
Lafdil, F. et al. Induction of Gas6 protein in CCl4-induced rat liver injury and anti-apoptotic effect on hepatic stellate cells. Hepatology 44, 228–239 (2006).
Novo, E. et al. Cellular and molecular mechanisms in liver fibrogenesis. Arch. Biochem. Biophys. 548, 20–37 (2014).
Chung, S. I. et al. Hepatic expression of Sonic Hedgehog induces liver fibrosis and promotes hepatocarcinogenesis in a transgenic mouse model. J. Hepatol. 64, 618–627 (2016).
Vaughn, B. P., Robson, S. C. & Longhi, M. S. Purinergic signaling in liver disease. Dig. Dis. 32, 516–524 (2014).
Wree, A. et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59, 898–910 (2014).
McHedlidze, T. et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 39, 357–371 (2013).
Canbay, A. et al. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab. Invest. 83, 655–663 (2003).
Zhan, S. S. et al. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo . Hepatology 43, 435–443 (2006).
Wiegard, C. et al. Murine liver antigen presenting cells control suppressor activity of CD4+CD25+ regulatory T cells. Hepatology 42, 193–199 (2005).
Seki, S. et al. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol. Rev. 174, 34–46 (2000).
Ju, C. & Pohl, L. R. Tolerogenic role of Kupffer cells in immune-mediated adverse drug reactions. Toxicology 209, 109–112 (2005).
Sunami, Y. et al. Hepatic activation of IKK/NFkappaB signaling induces liver fibrosis via macrophage-mediated chronic inflammation. Hepatology 56, 1117–1128 (2012).
Duffield, J. S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Pradere, J. P. et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology 58, 1461–1473 (2013).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Tacke, F. & Randolph, G. J. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 211, 609–618 (2006).
Seki, E. et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Invest. 119, 1858–1870 (2009).
Karlmark, K. R. et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274 (2009).
Miura, K., Yang, L., van Rooijen, N., Ohnishi, H. & Seki, E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1310–G1321 (2012).
Heinrichs, D. et al. The chemokine CCL3 promotes experimental liver fibrosis in mice. PLoS ONE 8, e66106 (2013).
Berres, M. L. et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J. Clin. Invest. 120, 4129–4140 (2010).
Fallowfield, J. A. et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 178, 5288–5295 (2007).
Pellicoro, A. et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology 55, 1965–1975 (2012).
Taimr, P. et al. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology 37, 87–95 (2003).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl Acad. Sci. USA 109, E3186–E3195 (2012).
Aoyama, T., Inokuchi, S., Brenner, D. A. & Seki, E. CX3CL1–CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 52, 1390–1400 (2010).
Jeong, W. I., Park, O. & Gao, B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology 134, 248–258 (2008).
Jeong, W. I. et al. Suppression of innate immunity (natural killer cell/interferon-gamma) in the advanced stages of liver fibrosis in mice. Hepatology 53, 1342–1351 (2011).
Glassner, A. et al. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab. Invest. 92, 967–977 (2012).
Wang, H. & Yin, S. Natural killer T cells in liver injury, inflammation and cancer. Expert Rev. Gastroenterol. Hepatol. 9, 1077–1085 (2015).
Park, O. et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology 49, 1683–1694 (2009).
Wehr, A. et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J. Immunol. 190, 5226–5236 (2013).
Deleve, L. D., Wang, X. & Guo, Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 48, 920–930 (2008).
Xie, G. et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 142, 918–927.e6 (2012).
Ding, B. S. et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505, 97–102 (2014).
Kurokawa, T., Zheng, Y. W. & Ohkohchi, N. Novel functions of platelets in the liver. J. Gastroenterol. Hepatol. 31, 745–751 (2016).
Nowatari, T., Murata, S., Fukunaga, K. & Ohkohchi, N. Role of platelets in chronic liver disease and acute liver injury. Hepatol. Res. 44, 165–172 (2014).
Yoshida, S. et al. Extrahepatic platelet-derived growth factor-beta, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 147, 1378–1392 (2014).
Novobrantseva, T. I. et al. Attenuated liver fibrosis in the absence of B cells. J. Clin. Invest. 115, 3072–3082 (2005).
Thapa, M. et al. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology 61, 2067–2079 (2015).
Pellicoro, A., Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 14, 181–194 (2014).
Kendall, T. J. et al. p75 neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology 49, 901–910 (2009).
Oh, Y. et al. Systemic PEGylated TRAIL treatment ameliorates liver cirrhosis in rats by eliminating activated hepatic stellate cells. Hepatology 64, 209–223 (2016).
Murphy, F. R. et al. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J. Biol. Chem. 277, 11069–11076 (2002).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Yoon, Y. J., Friedman, S. L. & Lee, Y. A. Antifibrotic therapies: where are we now? Semin. Liver Dis. 36, 87–98 (2016).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease — meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Lade, A., Noon, L. A. & Friedman, S. L. Contributions of metabolic dysregulation and inflammation to nonalcoholic steatohepatitis, hepatic fibrosis, and cancer. Curr. Opin. Oncol. 26, 100–107 (2014).
Bian, Z. & Ma, X. Liver fibrogenesis in non-alcoholic steatohepatitis. Front. Physiol. 3, 248 (2012).
Sanyal, A. J. et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1–5 (2010).
Verbeke, L. et al. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci. Rep. 6, 33453 (2016).
Friedman, S. et al. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR phase 2b study design. Contemp. Clin. Trials 47, 356–365 (2016).
Liu, S. B. et al. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 30, 1599–1609 (2016).
Barry-Hamilton, V. et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16, 1009–1017 (2010).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT00063622 (2012).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT00501592 (2012).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT01694849 (2016).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT02217475 (2016).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT01672866 (2017).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT01672879 (2017).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT02421094 (2017).
US National Library of Medicine. ClinicalTrials.gov [online] https://clinicaltrials.gov/ct2/show/NCT02227459 (2016).
Acknowledgements
The laboratory of S.L.F. is supported by NIH grant DK56621.
Author information
Authors and Affiliations
Contributions
Both authors discussed the concepts and contributed to the content of the work. T.T. generated a first draft of the article and S.L.F. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.L.F. is a consultant to 3-V Biotherapeutics, Abbvie Pharmaceuticals, Angion Biomedica, Akarna Therapeutics, Blade Therapeutics, Blueprint medicines, Boehringer Ingelheim, Bristol Myers Squibb, Can-Fite Biopharma, Chemocentryx, Conatus, Daichi Sankyo, Debio Pharmaceuticals, Deerfield consulting, DeuteRx, DS Biosciences, Eli Lilly Pharmaceuticals, Enanta Pharmaceuticals, Exalenz Biosciences, Fibrogen, Fractyl Bioscience, Galectin Therapeutics, Galmed, Genfit, Genkyotex, Glycotest, Glympse Bio, GNI Group, Immune Pharmaceuticals, Intercept, Ironwood Pharmaceuticals, Isis Pharmaceuticals, Janssen Pharmaceuticals, Jecure Therapeutics, Madrigal Pharmaceuticals, Metacrine, Metagenix, Merck Pharmaceuticals, Nimbus Therapeutics, Nitto, Northern Biologics, Novartis, Ocera Therapeutics, Pfizer, Raptor Pharmaceuticals, Roche, RuiYi, Sandhill Medical Devices, Scholar Rock, Shire Pharmaceuticals, Synageva BioPharma, Takeda Pharmaceuticals, Teva Pharmaceuticals, Tobira Therapeutics, Tokai Pharmaceuticals, Viking Therapeutics and Zafgen. T.T. is an employee of Mitsubushi Tanabe Pharma, Japan.
Rights and permissions
About this article
Cite this article
Tsuchida, T., Friedman, S. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14, 397–411 (2017). https://doi.org/10.1038/nrgastro.2017.38
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2017.38
This article is cited by
-
MyD88 in myofibroblasts enhances nonalcoholic fatty liver disease-related hepatocarcinogenesis via promoting macrophage M2 polarization
Cell Communication and Signaling (2024)
-
TC14012 enhances the anti-fibrosis effects of UC-MSCs on the liver by reducing collagen accumulation and ameliorating inflammation
Stem Cell Research & Therapy (2024)
-
Exploration of a hypoxia-immune-related microenvironment gene signature and prediction model for hepatitis C-induced early-stage fibrosis
Journal of Translational Medicine (2024)
-
Cell senescence in liver diseases: pathological mechanism and theranostic opportunity
Nature Reviews Gastroenterology & Hepatology (2024)
-
Activated hepatic stellate cell-derived Bmp-1 induces liver fibrosis via mediating hepatocyte epithelial-mesenchymal transition
Cell Death & Disease (2024)