Key Points
-
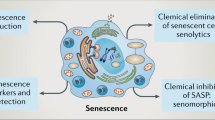
Senescence is a durable cell cycle arrest induced in response to various stress factors such as telomere erosion, DNA damage or the aberrant activation of oncogenes; the senescence response counteracts tumorigenesis
-
Senescence plays important physiological roles in nondisease settings such as embryonic development, wound healing, tissue repair and ageing
-
Senescent cells secrete various cytokines, chemokines, matrix remodelling proteases and growth factors, forming the senescence-associated secretory phenotype
-
Senescence-associated secreted factors evoke immune responses, which, depending on the pathophysiological context, can either prevent or fuel disease progression
-
Senescence plays a key part in the pathogenesis of many gastrointestinal and hepatobiliary diseases
Abstract
Senescence is a durable cell cycle arrest that can be induced in response to various stress factors, such as telomere erosion, DNA damage or the aberrant activation of oncogenes. In addition to its well-established role as a stress response programme, research has revealed important physiological roles of senescence in nondisease settings, such as embryonic development, wound healing, tissue repair and ageing. Senescent cells secrete various cytokines, chemokines, matrix remodelling proteases and growth factors, a phenotype collectively referred to as the senescence-associated secretory phenotype. These factors evoke immune responses that, depending on the pathophysiological context, can either prevent or even fuel disease and tumorigenesis. Remarkably, even the gut microbiota can influence senescence in various organs. In this Review, we provide an introduction to cellular senescence, addressed particularly to gastroenterologists and hepatologists, and discuss the implications of senescence for the pathogenesis of malignant and nonmalignant gastrointestinal and hepatobiliary diseases. We conclude with an outlook on how modulation of cellular senescence might be used for therapeutic purposes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
Munoz-Espin, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014).
Kuilman, T., Michaloglou, C., Mooi, W. J. & Peeper, D. S. The essence of senescence. Genes Dev. 24, 2463–2479 (2010).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Munoz-Espin, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Serrano, M. Senescence helps regeneration. Dev. Cell 31, 671–672 (2014).
Ritschka, B. et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 31, 172–183 (2017).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013).
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005).
Eggert, T. et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell 30, 533–547 (2016).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Campisi, J. & d'Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Nardella, C., Clohessy, J. G., Alimonti, A. & Pandolfi, P. P. Pro-senescence therapy for cancer treatment. Nat. Rev. Cancer 11, 503–511 (2011).
Cadoo, K. A., Gucalp, A. & Traina, T. A. Palbociclib: an evidence-based review of its potential in the treatment of breast cancer. Breast Cancer 6, 123–133 (2014).
Yoshida, A., Lee, E. K. & Diehl, J. A. Induction of therapeutic senescence in vemurafenib-resistant melanoma by extended inhibition of CDK4/6. Cancer Res. 76, 2990–3002 (2016).
Rader, J. et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin. Cancer Res. 19, 6173–6182 (2013).
Sherr, C. J. A new cell-cycle target in cancer — inhibiting cyclin D-dependent kinases 4 and 6. N. Engl. J. Med. 375, 1920–1923 (2016).
Acosta, J. C. & Gil, J. Senescence: a new weapon for cancer therapy. Trends Cell Biol. 22, 211–219 (2012).
Dabritz, J. H. et al. CD20-targeting immunotherapy promotes cellular senescence in B-cell lymphoma. Mol. Cancer Ther. 15, 1074–1081 (2016).
Collado, M. & Serrano, M. The power and the promise of oncogene-induced senescence markers. Nat. Rev. Cancer 6, 472–476 (2006).
Kuilman, T. & Peeper, D. S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 9, 81–94 (2009).
Sharpless, N. E. & Sherr, C. J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 15, 397–408 (2015).
Kurz, D. J., Decary, S., Hong, Y. & Erusalimsky, J. D. Senescence-associated β-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 113, 3613–3622 (2000).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Lee, B. Y. et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5, 187–195 (2006).
Freund, A., Laberge, R. M., Demaria, M. & Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 23, 2066–2075 (2012).
Shimi, T. et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 25, 2579–2593 (2011).
Anwar, T., Khosla, S. & Ramakrishna, G. Increased expression of SIRT2 is a novel marker of cellular senescence and is dependent on wild type p53 status. Cell Cycle 15, 1883–1897 (2016).
Althubiti, M. et al. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. 5, e1528 (2014).
Dörr, J. R. et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425 (2013).
Jiang, P., Du, W., Mancuso, A., Wellen, K. E. & Yang, X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 493, 689–693 (2013).
Kaplon, J. et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 498, 109–112 (2013).
Sun, N., Youle, R. J. & Finkel, T. The mitochondrial basis of aging. Mol. Cell 61, 654–666 (2016).
Nacarelli, T. & Sell, C. Targeting metabolism in cellular senescence, a role for intervention. Mol. Cell. Endocrinol. 455, 83–92 (2016).
Wiley, C. D. & Campisi, J. From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab. 23, 1013–1021 (2016).
Deng, Y., Chan, S. S. & Chang, S. Telomere dysfunction and tumour suppression: the senescence connection. Nat. Rev. Cancer 8, 450–458 (2008).
Salama, R., Sadaie, M., Hoare, M. & Narita, M. Cellular senescence and its effector programs. Genes Dev. 28, 99–114 (2014).
van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Roos, W. P., Thomas, A. D. & Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 16, 20–33 (2015).
Pearl, L. H., Schierz, A. C., Ward, S. E., Al-Lazikani, B. & Pearl, F. M. G. Therapeutic opportunities within the DNA damage response. Nat. Rev. Cancer 15, 166–180 (2015).
Fumagalli, M. et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 14, 355–365 (2012).
Banito, A. & Lowe, S. W. A new development in senescence. Cell 155, 977–978 (2013).
Mosteiro, L. et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354, aaf4445 (2016).
Rodier, F. in Cell Senescence: Methods and Protocols (eds Galluzzi, L., Vitale, I., Kepp, O. & Kroemer, G.) 165–173 (Humana Press, 2013).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
Schneider, C. et al. Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer. Gut 61, 1733–1743 (2012).
Toso, A. et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9, 75–89 (2014).
Di Mitri, D. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Aravinthan, A. D. & Alexander, G. J. Senescence in chronic liver disease: is the future in aging? J. Hepatol. 65, 825–834 (2016).
Meng, L. et al. Functional role of cellular senescence in biliary injury. Am. J. Pathol. 185, 602–609 (2015).
Sasaki, M. et al. Bile ductular cells undergoing cellular senescence increase in chronic liver diseases along with fibrous progression. Am. J. Clin. Pathol. 133, 212–223 (2010).
Nakanuma, Y., Sasaki, M. & Harada, K. Autophagy and senescence in fibrosing cholangiopathies. J. Hepatol. 62, 934–945 (2015).
Tabibian, J. H., O'Hara, S. P., Splinter, P. L., Trussoni, C. E. & LaRusso, N. F. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology 59, 2263–2275 (2014).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Aravinthan, A. et al. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J. Hepatol. 58, 549–556 (2013).
Piscaglia, F. et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 63, 827–838 (2016).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Schafer, M. J. et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 8, 14532 (2017).
Lunz, J. G., 3rd et al. Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: increased expression of p21WAF1/Cip1 as a disease marker and the influence of immunosuppressive drugs. Am. J. Pathol. 158, 1379–1390 (2001).
Brain, J. G. et al. Biliary epithelial senescence and plasticity in acute cellular rejection. Am. J. Transplant 13, 1688–1702 (2013).
Tabibian, J. H. & Lindor, K. D. Primary sclerosing cholangitis: a review and update on therapeutic developments. Expert Rev. Gastroenterol. Hepatol. 7, 103–114 (2013).
Sasaki, M. & Nakanuma, Y. Cellular senescence in biliary pathology. Special emphasis on expression of a polycomb group protein EZH2 and a senescent marker p16INK4a in bile ductular tumors and lesions. Histol. Histopathol. 30, 267–275 (2015).
Lasry, A. & Ben-Neriah, Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 36, 217–228 (2015).
Rodier, F. et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11, 973–979 (2009).
Rudolph, K. L., Chang, S., Millard, M., Schreiber-Agus, N. & DePinho, R. A. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 287, 1253–1258 (2000).
Paradis, V. et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum. Pathol. 32, 327–332 (2001).
Wiemann, S. U. et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 16, 935–942 (2002).
Plentz, R. R. et al. Telomere shortening and inactivation of cell cycle checkpoints characterize human hepatocarcinogenesis. Hepatology 45, 968–976 (2007).
Sasaki, M., Ikeda, H., Yamaguchi, J., Nakada, S. & Nakanuma, Y. Telomere shortening in the damaged small bile ducts in primary biliary cirrhosis reflects ongoing cellular senescence. Hepatology 48, 186–195 (2008).
Farazi, P. A. et al. Differential impact of telomere dysfunction on initiation and progression of hepatocellular carcinoma. Cancer Res. 63, 5021–5027 (2003).
Nault, J. C. & Zucman-Rossi, J. TERT promoter mutations in primary liver tumors. Clin. Res. Hepatol. Gastroenterol. 40, 9–14 (2016).
Jäger, K. & Walter, M. Therapeutic targeting of telomerase. Genes 7, 39 (2016).
Middleton, G. et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 15, 829–840 (2014).
Greten, T. F. et al. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer 10, 209 (2010).
Fenoglio, D. et al. A multi-peptide, dual-adjuvant telomerase vaccine (GX301) is highly immunogenic in patients with prostate and renal cancer. Cancer Immunol. Immunother. 62, 1041–1052 (2013).
Salloum, R. et al. A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: a pediatric brain tumor consortium study. J. Neurooncol. 129, 443–451 (2016).
Janknecht, R. On the road to immortality: hTERT upregulation in cancer cells. FEBS Lett. 564, 9–13 (2004).
Pal, J., Gold, J. S., Munshi, N. C. & Shammas, M. A. Biology of telomeres: importance in etiology of esophageal cancer and as therapeutic target. Transl Res. 162, 364–370 (2013).
Morales, C. P., Lee, E. L. & Shay, J. W. In situ hybridization for the detection of telomerase RNA in the progression from Barrett's esophagus to esophageal adenocarcinoma. Cancer 83, 652–659 (1998).
Shammas, M. A. et al. Telomere maintenance in laser capture microdissection-purified Barrett's adenocarcinoma cells and effect of telomerase inhibition in vivo. Clin. Cancer Res. 14, 4971–4980 (2008).
Tefferi, A. et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N. Engl. J. Med. 373, 908–919 (2015).
Baerlocher, G. M. et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. N. Engl. J. Med. 373, 920–928 (2015).
Dunham, M. A., Neumann, A. A., Fasching, C. L. & Reddel, R. R. Telomere maintenance by recombination in human cells. Nat. Genet. 26, 447–450 (2000).
Lu, R. et al. Targeting homologous recombination and telomerase in Barrett's adenocarcinoma: impact on telomere maintenance, genomic instability and tumor growth. Oncogene 33, 1495–1505 (2014).
Larsson, L. G. Oncogene- and tumor suppressor gene-mediated suppression of cellular senescence. Semin. Cancer Biol. 21, 367–376 (2011).
Snover, D. C. Update on the serrated pathway to colorectal carcinoma. Hum. Pathol. 42, 1–10 (2011).
Leggett, B. & Whitehall, V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 138, 2088–2100 (2010).
De Sousa, E. M. F. et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat. Med. 19, 614–618 (2013).
Bennecke, M. et al. Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell 18, 135–146 (2010).
Carragher, L. A. et al. V600EBraf induces gastrointestinal crypt senescence and promotes tumour progression through enhanced CpG methylation of p16INK4a. EMBO Mol. Med. 2, 458–471 (2010).
Daskalakis, M. et al. Demethylation of a hypermethylated P15/INK4B gene in patients with myelodysplastic syndrome by 5-Aza-2'-deoxycytidine (decitabine) treatment. Blood 100, 2957–2964 (2002).
Kantarjian, H. et al. Decitabine improves patient outcomes in myelodysplastic syndromes. Cancer 106, 1794–1803 (2006).
Foersch, S. et al. VEGFR2 signaling prevents colorectal cancer cell senescence to promote tumorigenesis in mice with colitis. Gastroenterology 149, 177–189 (2015).
Distler, M. et al. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. BioMed Res. Int. 2014, 474905 (2014).
Hruban, R. H. et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am. J. Surg. Pathol. 28, 977–987 (2004).
Ag Moir, J., A. White, S. & Mann, J. Arrested development and the great escape – the role of cellular senescence in pancreatic cancer. Int. J. Biochem. Cell Biol. 57, 142–148 (2014).
Singh, S. K. & Ellenrieder, V. Senescence in pancreatic carcinogenesis: from signalling to chromatin remodelling and epigenetics. Gut 62, 1364–1372 (2013).
Makohon-Moore, A. & Iacobuzio-Donahue, C. A. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat. Rev. Cancer 16, 553–565 (2016).
Morton, J. P. et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc. Natl Acad. Sci. USA 107, 246–251 (2010).
De Nicola, G. M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109 (2011).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 (2009).
Kim, J., Kim, J. & Bae, J. S. ROS homeostasis and metabolism: a critical liaison for cancer therapy. Exp. Mol. Med. 48, e269 (2016).
Chio, I. I. C. et al. NRF2 promotes tumor maintenance by modulating mRNA translation in pancreatic cancer. Cell 166, 963–976 (2016).
Guerra, C. et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 19, 728–739 (2011).
Lowenfels, A. B. & Maisonneuve, P. Epidemiology and risk factors for pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 20, 197–209 (2006).
Wolfgang, C. L. et al. Recent progress in pancreatic cancer. CA Cancer J. Clin. 63, 318–348 (2013).
Lowery, M. A. & O'Reilly, E. M. Treatment of metastatic pancreatic adenocarcinoma: new options and promising strategies. Oncology 28 (2014).
Carriere, C. et al. Deletion of Rb accelerates pancreatic carcinogenesis by oncogenic Kras and impairs senescence in premalignant lesions. Gastroenterology 141, 1091–1101 (2011).
Rielland, M. et al. Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression. J. Clin. Invest. 124, 2125–2135 (2014).
Grandinetti, K. B. et al. Sin3B expression is required for cellular senescence and is up-regulated upon oncogenic stress. Cancer Res. 69, 6430–6437 (2009).
Ko, A. et al. Acceleration of gastric tumorigenesis through MKRN1-mediated posttranslational regulation of p14ARF. J. Natl Cancer Inst. 104, 1660–1672 (2012).
Zhang, X.-W. et al. UBTD1 induces cellular senescence through an UBTD1–Mdm2/p53 positive feedback loop. J. Pathol. 235, 656–667 (2015).
Zitvogel, L. et al. Cancer and the gut microbiota: an unexpected link. Sci. Transl Med. 7, 271ps1 (2015).
Lynch, S. V. & Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379 (2016).
Cougnoux, A. et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 63, 1932–1942 (2014).
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004).
Cuevas-Ramos, G. et al. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl Acad. Sci. 107, 11537–11542 (2010).
Arthur, J. C. et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012).
Daniel, H. et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 8, 295–308 (2014).
Ohtani, N., Yoshimoto, S. & Hara, E. Obesity and cancer: a gut microbial connection. Cancer Res. 74, 1885–1889 (2014).
Cox, A. J., West, N. P. & Cripps, A. W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 3, 207–215 (2015).
Ridlon, J. M., Kang, D.-J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
Kitazawa, S. et al. Enhanced preneoplastic liver lesion development under 'selection pressure' conditions after administration of deoxycholic or lithocholic acid in the initiation phase in rats. Carcinogenesis 11, 1323–1328 (1990).
Payne, C. M. et al. Deoxycholate induces mitochondrial oxidative stress and activates NF-κB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis 28, 215–222 (2006).
Polk, D. B. & Peek, R. M. Jr. Helicobacter pylori: gastric cancer and beyond. Nat. Rev. Cancer 10, 403–414 (2010).
Saito, Y., Murata-Kamiya, N., Hirayama, T., Ohba, Y. & Hatakeyama, M. Conversion of Helicobacter pylori CagA from senescence inducer to oncogenic driver through polarity-dependent regulation of p21. J. Exp. Med. 207, 2157–2174 (2010).
Schmitt, C. A. et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109, 335–346 (2002).
Haugstetter, A. M. et al. Cellular senescence predicts treatment outcome in metastasised colorectal cancer. Br. J. Cancer 103, 505–509 (2010).
te Poele, R. H., Okorokov, A. L., Jardine, L., Cummings, J. & Joel, S. P. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 62, 1876–1883 (2002).
Cairney, C. J. et al. Cancer cell senescence: a new frontier in drug development. Drug Discov. Today 17, 269–276 (2012).
Perez-Mancera, P. A., Young, A. R. J. & Narita, M. Inside and out: the activities of senescence in cancer. Nat. Rev. Cancer 14, 547–558 (2014).
Venturelli, S. et al. Differential induction of apoptosis and senescence by the DNA methyltransferase inhibitors 5-azacytidine and 5-aza-2′-deoxycytidine in solid tumor cells. Mol. Cancer Ther. 12, 2226–2236 (2013).
Ewald, J. A., Desotelle, J. A., Wilding, G. & Jarrard, D. F. Therapy-induced senescence in cancer. J. Natl Cancer Inst. 102, 1536–1546 (2010).
Venturelli, S. et al. Dual antitumour effect of 5-azacytidine by inducing a breakdown of resistance-mediating factors and epigenetic modulation. Gut 60, 156–165 (2011).
Weiland, T. et al. Enhanced killing of therapy-induced senescent tumor cells by oncolytic measles vaccine viruses. Int. J. Cancer 134, 235–243 (2014).
Vu, B. et al. Discovery of RG7112: a small-molecule MDM2 inhibitor in clinical development. ACS Med. Chem. Lett. 4, 466–469 (2013).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Postow, M. A., Callahan, M. K. & Wolchok, J. D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 33, 1974–1982 (2015).
Braumuller, H. et al. T-Helper-1-cell cytokines drive cancer into senescence. Nature 494, 361–365 (2013).
Zecchini, V. & Frezza, C. Metabolic synthetic lethality in cancer therapy. Biochim. Biophys. Acta 1858, 723–731 (2017).
Di Mitri, D. & Alimonti, A. Non-cell-autonomous regulation of cellular senescence in cancer. Trends Cell Biol. 26, 215–226 (2016).
Wu, P. C., Wang, Q., Grobman, L., Chu, E. & Wu, D. Y. Accelerated cellular senescence in solid tumor therapy. Exp. Oncol. 34, 298–305 (2012).
Pribluda, A. et al. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell 24, 242–256 (2013).
Elyada, E. et al. CKIα ablation highlights a critical role for p53 in invasiveness control. Nature 470, 409–413 (2011).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Zhu, Y. et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147 (2017).
Duarte, L. F. et al. Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat. Commun. 5, 5210 (2014).
Acknowledgements
The authors' work was supported by the German Research Foundation (DFG) grants FOR2314 (L.Z.), SFB685 (L.Z.) and SFB/TR 209 (L.Z. and M.B.), the Gottfried Wilhelm Leibniz Program (L.Z.), the European Research Council (projects 'CholangioConcept' (L.Z.) and 'Heptromic' (L.Z.)), the German Ministry for Education and Research (BMBF) (eMed [Multiscale HCC]) (L.Z., M.B.), the German Universities Excellence Initiative (third funding line: 'future concept') (L.Z.), the German Centre for Translational Cancer Research (DKTK) (L.Z.) and the German-Israeli Cooperation in Cancer Research (DKFZ-MOST) (L.Z.).
Author information
Authors and Affiliations
Contributions
L.Z. and M.B. researched data for the article. M.B., L.Z. and N.F. wrote the manuscript. All authors discussed the outline and content of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Frey, N., Venturelli, S., Zender, L. et al. Cellular senescence in gastrointestinal diseases: from pathogenesis to therapeutics. Nat Rev Gastroenterol Hepatol 15, 81–95 (2018). https://doi.org/10.1038/nrgastro.2017.146
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2017.146
This article is cited by
-
Rejuvenating aged microglia by p16ink4a-siRNA-loaded nanoparticles increases amyloid-β clearance in animal models of Alzheimer’s disease
Molecular Neurodegeneration (2024)
-
Tumor-Immunzell-Interaktion und Seneszenz-assoziierte Moleküle im kolorektalen Karzinom
Die Pathologie (2023)
-
The role of cellular senescence in cardiac disease: basic biology and clinical relevance
Nature Reviews Cardiology (2022)
-
CD8+ T cell differentiation and dysfunction in cancer
Nature Reviews Immunology (2022)
-
When dormancy fuels tumour relapse
Communications Biology (2021)