Key Points
-
HCV prevalence and incidence among people who inject drugs (PWID) remains high
-
Direct-acting antiviral agents (DAA) for HCV with cure in >95% provide a tool for addressing HCV-related liver-disease burden among PWID
-
Adherence and response to DAA therapy among people receiving opioid substitution therapy (OST), including those with ongoing drug use, are comparable to other HCV-infected populations, although more data are required among current PWID who are not on OST
-
HCV reinfection can occur, so strategies to maximize prevention of reinfection (including OST and needle and syringe programmes) and access to DAA retreatment are crucial
-
Improved health services for PWID are needed to enhance HCV prevention, testing, access to care and treatment
-
Continued global leadership and advocacy is required to ensure that HCV prevention, care and treatment for PWID are accessible to all
Abstract
Globally, 12 million people are estimated to have injected drugs in the past year, 50% of whom have chronic HCV infection, with people who have previously injected drugs presenting an additional large reservoir of infection. The availability of simple and tolerable interferon-free direct-acting antiviral agents (DAAs) for HCV infection, which have a cure rate of >95% represents one of the most exciting advances in clinical medicine in the past few decades. Adherence and response to DAA therapy among people who inject drugs (PWID) receiving opioid substitution therapy (OST) in clinical trials are comparable to populations without a history of injecting drugs. Further data are required among current PWID not receiving OST. Given the potential prevention benefits of treatment, DAAs have enhanced cost-effectiveness among PWID. As HCV therapy is expanded to populations of PWID with high-risk behaviours for re-exposure, acknowledgement that HCV reinfection will occur is crucial, and appropriate strategies must be in place to maximize prevention of reinfection and offer retreatment for reinfection. This Review will also discuss essential components for broadening access to HCV care for PWID as we strive for the global elimination of HCV infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hajarizadeh, B., Grebely, J. & Dore, G. J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 10, 553–562 (2013).
Nelson, P. K. et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 378, 571–583 (2011).
Hellard, M., Sacks-Davis, R. & Gold, J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin. Infect. Dis. 49, 561–573 (2009).
Dimova, R. B. et al. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin. Infect. Dis. 56, 806–816 (2013).
Aspinall, E. J. et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin. Infect. Dis. 57 (Suppl. 2), S80–S89 (2013).
Grebely, J. et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int. J. Drug Policy 26, 1028–1038 (2015).
Alavi, M. et al. Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents. Liver Int. 34, 1198–1206 (2014).
Iversen, J. et al. Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia, 1999–2011. J. Viral Hepat. 21, 198–207 (2014).
Wiessing, L. et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS ONE 9, e103345 (2014).
Dore, G. J. & Feld, J. J. Hepatitis C virus therapeutic development: in pursuit of “perfectovir”. Clin. Infect. Dis. 60, 1829–1836 (2015).
Barua, S. et al. Restrictions for medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann. Intern. Med. 163, 215–223 (2015).
Asher, A. K. et al. Clinicians' views of hepatitis C virus treatment candidacy with direct-acting antiviral regimens for people who inject drugs. Subst. Use Misuse 51, 1218–1223 (2016).
Grebely, J., Oser, M., Taylor, L. E. & Dore, G. J. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J. Infect. Dis. 207 (Suppl. 1), S19–S25 (2013).
Larney, S. et al. Defining populations and injecting parameters among people who inject drugs: Implications for the assessment of hepatitis C treatment programs. Int. J. Drug Policy 26, 950–957 (2015).
UNODC. World Drug Report 2015. United Nations Office on Drugs and Crime http://www.unodc.org/wdr2015/ (2015).
Pybus, O. G., Cochrane, A., Holmes, E. C. & Simmonds, P. The hepatitis C virus epidemic among injecting drug users. Infect. Genet. Evol. 5, 131–139 (2005).
Robaeys, G. et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin. Infect. Dis. 57 (Suppl. 2), S129–S137 (2013).
van Asten, L. et al. Spread of hepatitis C virus among European injection drug users infected with HIV: a phylogenetic analysis. J. Infect. Dis. 189, 292–302 (2004).
Sievert, W. et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 31 (Suppl. 2), 61–80 (2011).
Hagan, H., Pouget, E. R., Des Jarlais, D. C. & Lelutiu-Weinberger, C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am. J. Epidemiol. 168, 1099–1109 (2008).
Page, K., Morris, M. D., Hahn, J. A., Maher, L. & Prins, M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin. Infect. Dis. 57 (Suppl. 2), S32–S38 (2013).
Roy, E., Boudreau, J. F. & Boivin, J. F. Hepatitis C virus incidence among young street-involved IDUs in relation to injection experience. Drug Alcohol Depend. 102, 158–116 (2009).
Pierce, B. G., Keck, Z. Y. & Foung, S. K. Viral evasion and challenges of hepatitis C virus vaccine development. Curr. Opin. Virol. 20, 55–63 (2016).
Swadling, L. et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci. Transl. Med. 6, 261ra153 (2014).
US National Library of Medicine. ClinitralTrial.gov https://clinicaltrials.gov/ct2/show/NCT01436357 (2017).
MacArthur, G. J. et al. Interventions to prevent HIV and hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int. J. Drug Policy 25, 34–52 (2014).
Degenhardt, L. et al. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet 376, 285–301 (2010).
Hagan, H., Pouget, E. R. & Des Jarlais, D. C. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J. Infect. Dis. 204, 74–83 (2011).
Turner, K. M. et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 106, 1978–1988 (2011).
van den Berg, C. H. et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. Eur. J. Epidemiol. 22, 183–193 (2007).
Nolan, S. et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 109, 2053–2059 (2014).
Aspinall, E. J. et al. Does informing people who inject drugs of their hepatitis C status influence their injecting behaviour? Analysis of the Networks II study. Int. J. Drug Policy 25, 179–182 (2014).
White, B., Dore, G. J., Lloyd, A. R., Rawlinson, W. D. & Maher, L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Med. J. Aust. 201, 326–329 (2014).
Tsui, J. I., Evans, J. L., Lum, P. J., Hahn, J. A. & Page, K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern. Med. 174, 1974–1981 (2014).
Martin, N. K. et al. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J. Hepatol. 54, 1137–1144 (2011).
Martin, N. K., Hickman, M., Hutchinson, S. J., Goldberg, D. J. & Vickerman, P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin. Infect. Dis. 57 (Suppl. 2), S39–S45 (2013).
Martin, N. K. et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 58, 1598–1609 (2013).
de Vos, A. S., Prins, M. & Kretzschmar, M. E. Hepatitis C virus treatment as prevention among injecting drug users: who should we cure first? Addiction 110, 975–983 (2015).
Hellard, M. et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology 60, 1861–1870 (2014).
Williams, R. et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 384, 1953–1997 (2014).
Martin, N. K. et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology 55, 49–57 (2012).
Martin, N. K. et al. Prioritization of HCV treatment in the direct-acting antiviral era: an economic evaluation. J. Hepatol. 65, 17–25 (2016).
AASLD and IDSA. HCV guidance: recommendations for testing, managing, and treating hepatitis C. AASLD and IDSA www.hcvguidelines.org (2015).
European Association for Study of Liver. EASL recommendations on treatment of hepatitis C 2015. J. Hepatol, 63, 199–236 (2015).
World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection (WHO, 2014).
Grebely, J. & Dore, G. J. What is killing people with hepatitis C virus infection? Semin. Liver Dis. 31, 331–339 (2011).
van der Meer, A. J. et al. Life expectancy in patients with chronic HCV infection and cirrhosis compared with a general population. JAMA 312, 1927–1928 (2014).
Smith, D. J., Combellick, J., Jordan, A. E. & Hagan, H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): a systematic review and meta-analysis. Int. J. Drug Policy 26, 911–921 (2015).
Puoti, M. et al. ABT-450/r/ombitasvir plus dasabuvir with or without ribavirin in HCV genotype 1-infected patients receiving stable opioid substitution treatment: pooled analysis of efficacy and safety in phase 2 and phase 3 trials. Hepatology 60, 1135a–1136a (2014).
Feld, J. J. et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N. Engl. J. Med. 370, 1594–1603 (2014).
Zeuzem, S. et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann. Internal Med. 163, 1–13 (2015).
Grebely, J. et al. Efficacy and safety of sofosbuvir/velpatasvir in patients with chronic hepatitis C virus infection receiving opioid substitution therapy: analysis of phase 3 ASTRAL trials. Clin. Infect. Dis. 63, 1479–1481 (2016).
Grebely, J. et al. Efficacy and safety of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic HCV genotype 1 infection receiving opioid substitution therapy: analysis of phase 3 ION trials. Clin. Infect. Dis. 63, 1405–1411 (2016).
Grebely, J. et al. Safety and efficacy of ombitasvir, paritaprevir/ritonavir and dasabuvir with or without ribavirin in chronic hepatitis C patients receiving opioid substitution therapy: a pooled analysis across 12 clinical trials [Abstract]. J. Hepatol. 66 (Suppl.), S514 (2017).
Grebely, J. et al. SOF/VEL/VOX for 8 or 12 weeks is well tolerated and results in high SVR12 rates in patients receiving opioid substitution therapy [Abstract]. J. Hepatol. 66 (Suppl.), S513 (2017).
Dore, G. J. et al. Elbasvir/grazoprevir to treat HCV infection in persons receiving opioid agonist therapy: a randomized controlled trial (C-EDGE CO-STAR). Ann. Internal Med. 165, 625–634 (2016).
Lalezari, J. et al. Ombitasvir/paritaprevir/r and dasabuvir plus ribavirin in HCV genotype 1-infected patients on methadone or buprenorphine. J. Hepatol. 63, 364–369 (2015).
Christensen, S. et al. DAA-treatment of HCV-infected patients on opioid substitution therapy (OST): does the clinical setting matter? Data from the german hepatitis C-Registry (DHC-R). Hepatology 64, 982A–983A (2016).
Schütz, A., Moser, S., Marchart, K., Haltmayer, H. & Gschwantler, M. Direct observed therapy of chronic hepatitis C with interferon-free all-oral regimens at a low-threshold drug treatment facility-a new concept for treatment of patients with borderline compliance receiving opioid substitution therapy. Am. J. Gastroenterol. 111, 903–905 (2016).
Scherz, N., Brunner, N. & Bruggmann, P. Direct-acting antivirals for hepatitis C in patient in opioid substitution treatment and heroin assisted treatment: real-life data. J. Hepatol. 66, S726 (2017).
Dillon, J. et al. Efficacy and safety of simeprevir-containing hepatitis C therapy in patients on opiate substitution therapy [Abstract]. J. Hepatol. 66, S520 (2017).
Moser, S. et al. Directly observed therapy with sofosbuvir/ledipasvir for 8 weeks is highly effective in treatment-naïve, precirrhotic genotype 1 patients with borderline compliance receiving opioid agonist therapy [Abstract]. J. Hepatol. 66, S740 (2017).
Boyle, A. et al. Partial directly observed therapy with ombitasvir/paritaprevir based regimens allows for successful treatment of patients on daily supervised methadone [Abstract]. J. Hepatol. 66, S282 (2017).
Norton, B. L. et al. High HCV cure rates for drug users treated with DAAs at an urban primary care clinic. Presented at Conference on Retroviruses and Opportunistic Infections.
Conway, B. et al. Efficacy of all-oral HCV therapy in people who inject drugs [Abstract #1992]. Hepatology 64, 990A (2016).
Morris, L. et al. Initial outcomes of integrated community-based hepatitis C treatment for people who inject drugs: findings from the Queensland Injectors' Health Network. Int. J. Drug Policy http://dx.doi.org/10.1016/j.drugpo.2017.05.056 (2017).
Read, P. et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int. Drug Policy http://dx.doi.org/10.1016/j.drugpo.2017.05.032 (2017).
Bouscaillou, J. et al. Effectiveness of DAA-based treatment of HCV in active people who inject drugs living in middle income countries (MIC): the results of a prospective cohort study in Tbilisi, Georgia [Abstract[. J. Hepatol. 66, S409 (2017).
Sulkowski, M. et al. Randomized controlled trial of cash incentives or peer mentors to improve HCV linkage and treatment among HIV/HCV coinfected persons who inject drugs: the CHAMPS Study [Abstract]. J. Hepatol. 66, S719 (2017).
Mason, K. et al. Understanding real-world adherence in the directly acting antiviral era: a prospective evaluation of adherence amongst people with a history of drug use at a community-based program in Toronto. Canada. Int. Drug Policy http://dx.doi.org/10.1016/j.drugpo.2017.05.02 (2017).
Boglione, L. et al. Treatment with direct-acting antiviral agents of hepatitis C virus infection in injecting drug users: a prospective study. J. Viral Hepat. http://dx.doi.org/10.1111/jvh.12711 (2017).
Grebely, J. et al. Efficacy and safety of sofosbuvir/velpatasvir in people with chronic hepatitis C virus infection and recent injecting drug use: the SIMPLIFY study [Abstract]. J. Hepatol. 66, S513 (2017).
Cunningham, E. B., Applegate, T. L., Lloyd, A. R., Dore, G. J. & Grebely, J. Mixed HCV infection and reinfection in people who inject drugs — impact on therapy. Nat. Gastroenterol. Hepatol. 12, 218–230 (2015).
Simmons, B., Saleem, J., Hill, A., Riley, R. D. & Cooke, G. S. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin. Infect dis 62, 683–694 (2016).
Midgard, H. et al. Hepatitis C reinfection after sustained virological response. J. Hepatol. 64, 1020–1026 (2016).
Weir, A. et al. Hepatitis C reinfection following treatment induced viral clearance among people who have injected drugs. Drug Alcohol Depend 165, 53–60 (2016).
Pineda, J. A. et al. Hepatitis C virus reinfection after sustained virological response in HIV-infected patients with chronic hepatitis C. J. Infect. 71, 571–577 (2015).
Alavi, M. et al. Injecting risk behaviours following treatment for hepatitis C virus infection among people who inject drugs: The Australian Trial in Acute Hepatitis C. Int. Drug Policy 26, 976–983 (2015).
Crawford, S. & Bath, N. Peer support models for people with a history of injecting drug use undertaking assessment and treatment for hepatitis C virus infection. Clin. Infect dis 57 (Suppl. 2), S75–S79 (2013).
Razavi, H. et al. Modelling the impact of hepatitis C virus (HCV) treatment as prevention among people who inject drugs (PWIDs) in Australia. Presented at the 4th International Symposium on Hepatitis in Substance Users.
Hellard, M. et al. Hepatitis C transmission and treatment as prevention - The role of the injecting network. Int. Drug Policy 26, 958–962 (2015).
Mathers, B. M. et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet 375, 1014–1028 (2010).
Grebely, J. & Dore, G. J. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res. 104, 62–72 (2014).
Saraswat, V. et al. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 2. J. Viral Hepatitis 22 (Suppl. 1), 6–25 (2015).
Liakina, V. et al. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 3. J. Viral Hepatitis 22 (Suppl. 4), 4–20 (2015).
Bruggmann, P. et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J. Viral Hepatitis 21 (Suppl. 1), 5–33 (2014).
Zhou, K. et al. Interventions to optimise the care continuum for chronic viral hepatitis: a systematic review and meta-analyses. Lancet Infect. Dis 16, 1409–1422 (2016).
Cullen, W. et al. Hepatitis C infection among injecting drug users in general practice: a cluster randomised controlled trial of clinical guidelines' implementation. Br. Pract. 56, 848–856 (2006).
Rosenberg, S. D. et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr. Serv. 61, 885–891 (2010).
Sahajian, F. et al. A randomized trial of viral hepatitis prevention among underprivileged people in the Lyon area of France. J. Publ. Health 33, 182–192 (2011).
Lacey, C., Ellen, S., Devlin, H., Wright, E. & Mijch, A. Hepatitis C in psychiatry inpatients: testing rates, prevalence and risk behaviours. Australas. Psychiatry 15, 315–319 (2007).
Meyer, J. P. et al. Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic hepatitis C care continuum. Int. Drug Policy 26, 922–935 (2015).
Krauskopf, K. et al. Evaluation of an electronic health record prompt for hepatitis c antibody screening of baby boomers in primary care-a cluster randomized control trial. J. Gen. Intern. Med. 29, S88–S89 (2014).
Litwin, A. H. et al. Primary care-based interventions are associated with increases in hepatitis C virus testing for patients at risk. Dig. Liver dis 44, 497–503 (2012).
Drainoni, M. L. et al. Effectiveness of a risk screener in identifying hepatitis C virus in a primary care setting. Am. Publ. Health 102, e115–e121 (2012).
Hickman, M. et al. Increasing the uptake of hepatitis C virus testing among injecting drug users in specialist drug treatment and prison settings by using dried blood spots for diagnostic testing: a cluster randomized controlled trial. J. Viral Hepatitis 15, 250–254 (2008).
Abou-Saleh, M. T., Rice, P. & Foley, S. Hepatitis C testing in drug users using the dried blood spot test and the uptake of an innovative self-administered DBS test. Addictive Disord. 12, 40–49 (2013).
Tait, J. M., Stephens, B. P., McIntyre, P., Evans, M. & Dillon, J. F. Dry blood spot testing for hepatitis C in people who injected drugs: reaching the populations other tests cannot reach. J. Hepatol. 58, S204–S204 (2013).
Craine, N., Parry, J., O'Toole, J., D'Arcy, S. & Lyons, M. Improving blood-borne viral diagnosis; clinical audit of the uptake of dried blood spot testing offered by a substance misuse service. J. Viral Hepatitis 16, 219–222 (2009).
McLeod, A. et al. Rise in testing and diagnosis associated with Scotland's Action Plan on Hepatitis C and introduction of dried blood spot testing. J. Epidemiol. Commun. Health 68, 1182–1188 (2014).
Coats, J. T. & Dillon, J. F. The effect of introducing point-of-care or dried blood spot analysis on the uptake of hepatitis C virus testing in high-risk populations: a systematic review of the literature. Int. Drug Policy 26, 1050–1055 (2015).
Morano, J. P. et al. Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic. J. Commun. Health 39, 922–934 (2014).
Bottero, J. et al. Simultaneous human immunodeficiency virus-hepatitis B-hepatitis C point-of-care tests improve outcomes in linkage-to-care: results of a randomized control trial in persons without healthcare coverage. Open Forum Infect. Dis. 2, ofv162 (2015).
Beckwith, C. G. et al. A pilot study of rapid hepatitis C virus testing in the Rhode Island Department of Corrections. J. Publ. Health 38, 130–137 (2016).
Terrence Higgins Trust. FASTEST direct. Terrence Higgins Trust https://www.tht.org.uk/sexual-health/About-HIV/HIV-postal-test (2016).
Wong, V. W. et al. Targeted hepatitis C screening among ex-injection drug users in the community. J. Gastroenterol. Hepatol. 29, 116–120 (2014).
Jewett, A. et al. Field-based performance of three pre-market rapid hepatitis C virus antibody assays in STAHR (Study to Assess Hepatitis C Risk) among young adults who inject drugs in San Diego, CA. J. Clin. Virol. 54, 213–217 (2012).
Smith, B. D. et al. Evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus. J. Infect. Dis. 204, 825–831 (2011).
Smith, B. D. et al. Performance of premarket rapid hepatitis C virus antibody assays in 4 national human immunodeficiency virus behavioral surveillance system sites. Clin. Infect. Dis. 53, 780–786 (2011).
Drobnik, A. et al. Public health implications of rapid hepatitis C screening with an oral swab for community-based organizations serving high-risk populations. Am. Publ. Health 101, 2151–2155 (2011).
Grebely, J. et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 59, 109–120 (2014).
UNITAID. Hepatitis C diagnostics technology landscape UNITAID http://unitaid.eu/assets/UNITAID-HCV_Diagnostic_Landscape-1st_edition.pdf (2015).
Grebely, J. et al. Evaluation of the Xpert® HCV viral load point-of-care assay from venipuncture-collected and finger-stick capillary whole-blood samples: a prospective study. Lancet Gastro Hepatol. 2, 514–520 (2017).
Freiman, J. M. et al. Hepatitis C core antigen testing for diagnosis of hepatitis C virus infection: a systematic review and meta-analysis. Ann. Intern. med. 165, 345–355 (2016).
Cohn, J., Roberts, T., Amorosa, V., Lemoine, M. & Hill, A. Simplified diagnostic monitoring for hepatitis C, in the new era of direct-acting antiviral treatment. Curr. Opin. HIV AIDS 10, 369–373 (2015).
McAllister, G. et al. Uptake of hepatitis C specialist services and treatment following diagnosis by dried blood spot in Scotland. J. Clin. Virol. 61, 359–364 (2014).
Moessner, B. K. et al. Outreach screening of drug users for cirrhosis with transient elastography. Addiction 106, 970–976 (2011).
Foucher, J. et al. FibroScan used in street-based outreach for drug users is useful for hepatitis C virus screening and management: a prospective study. J. Viral Hepatitis 16, 121–131 (2009).
Marshall, A. D. et al. Liver disease knowledge and acceptability of non-invasive liver fibrosis assessment among people who inject drugs in the drug and alcohol setting: The LiveRLife Study. Int. Drug Policy 26, 984–991 (2015).
Masson, C. L. et al. A randomized trial of a hepatitis care coordination model in methadone maintenance treatment. Am. Publ. Health 103, E81–E88 (2013).
Evon, D. M. et al. A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. Am. Gastroenterol. 106, 1777–1786 (2011).
Knott, A. et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am. Gastroenterol. 101, 2254–2262 (2006).
Ho, S. B. et al. Integrated care increases treatment and improves outcomes of patients with chronic hepatitis C virus infection and psychiatric illness or substance abuse. Clin. Gastroenterol. Hepatol. 13, 2005–2014e3 (2015).
Trooskin, S. B. et al. Results from a geographically focused, community-based HCV screening, linkage-to-care and patient navigation program. J. Gen. Intern. Med. 30, 950–957 (2015).
Falade-Nwulia, O. et al. Public health clinic-based hepatitis C testing and linkage to care in baltimore. J. Viral Hepatitis 23, 366–374 (2016).
Bruggmann, P. & Litwin, A. H. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin. Infect. Dis. 57 (Suppl. 2), S56–S61 (2013).
Platt, L. et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect dis 16, 797–808 (2016).
Grebely, J. et al. Excluding people who use drugs or alcohol from access to hepatitis C treatments - Is this fair, given the available data? J. Hepatol. 63, 779–782 (2015).
Marshall, A. D. et al. A review of restrictions for reimbursement of direct-acting antiviral treatment for hepatitis C virus infection in Canada. CMAJ Open 4, E605–E614 (2016).
Graham, J. Medicaid, private insurers begin to lift curbs on pricey hepatitis C drugs. Kaiser Health News http://khn.org/news/medicaid-private-insurers-begin-to-lift-curbs-on-pricey-hepatitis-c-drugs/ (2016).
The Kirby Institute. Monitoring hepatitis C treatment uptake in Australia (Issue 7). Kirby Institute https://kirby.unsw.edu.au/sites/default/files/kirby/report/Monitoring-hep-C-treatment-uptake-in-Australia_Iss7-JUL17.pdf (2017).
Aleccia, J. Judge orders Washington Medicaid to provide lifesaving hepatitis C drugs for all. The Seattle Times http://www.seattletimes.com/seattle-news/health/judge-orders-apple-health-to-cover-hepatitis-c-drugs-for-all/ (2016).
Jones, S. Jones introduces legislation to treat hepatitis C sooner. Sylvia Jones MPP http://sylviajonesmpp.ca/2016/06/08/jones-introduces-legislation-to-treat-hepatitis-c-sooner/ (2016).
Keats, J. et al. Assessment and delivery of treatment for hepatitis C virus infection in an opioid substitution treatment clinic with integrated peer-based support in Newcastle. Australia. Int. Drug Policy 26, 999–1006 (2015).
Myles, A., Mugford, G. J., Zhao, J., Krahn, M. & Wang, P. P. Physicians' attitudes and practice toward treating injection drug users with hepatitis C: results from a national specialist survey in Canada. Can. Gastroenterol. 25, 135–139 (2011).
Lambert, S. M. et al. General practitioner attitudes to prescribing hepatitis C antiviral therapy in a community setting. Aust. Prim. Health 17, 282–287 (2011).
World Health Organization. Global health sector strategies - Viral hepatitis, 2016–2021 (WHO, 2016).
Dolan, K. et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet 388, 1089–1102 (2016).
Altice, F. L. et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet 388, 1228–1248 (2016).
Butner, J. L. et al. Onsite treatment of HCV infection with direct acting antivirals within an opioid treatment program. J. Subst. Abuse Treat. 75, 49–53 (2017).
Litwin, A. H. et al. The PREVAIL Study: intensive models of HCV care for people who inject drugs J. Hepatol. 66, S72 (2017).
Acknowledgements
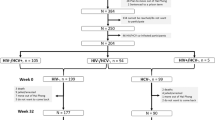
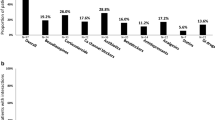
The authors would like to thank H. Razavi. Center for Disease Analysis, for providing the image for Fig. 2. J.G. acknowledges grant support from the NIH R01 DA 040506–01 and G.J.D. acknowledges grant support from RO1 DA 15999–01 and R01 DA 040506–01.
Author information
Authors and Affiliations
Contributions
J.G., B.H. and G.J.D. researched data for the article, contributed to discussion of content and reviewed and edited the manuscript before submission. J.G. wrote the first draft of the article.
Corresponding author
Ethics declarations
Competing interests
J.G. is a consultant and advisor, and has received research grants from AbbVie, Bristol-Myers Squibb, Cepheid, Gilead Sciences, Merck and Merck Sharp & Dohme. G.J.D. is a consultant and advisor, and has received research grants from Abbvie, Bristol–Myers Squibb, Cepheid, Gilead Sciences, Janssen Pharmaceuticals, Merck and Roche.
Supplementary information
Supplementary information S1 (table)
Baseline participant characteristics and treatment outcomes following DAA HCV treatment studies among people with a history of injecting drug use (PDF 165 kb)
Rights and permissions
About this article
Cite this article
Grebely, J., Hajarizadeh, B. & Dore, G. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol 14, 641–651 (2017). https://doi.org/10.1038/nrgastro.2017.106
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2017.106
This article is cited by
-
Prevalence, risk factors, treatment uptake and treatment outcome of hepatitis C virus in people who inject drugs at the needle and syringe program in Uppsala, Sweden
Harm Reduction Journal (2023)
-
Real-world hepatitis C treatment outcomes and reinfections among people who inject drugs at a needle and syringe program in Stockholm, Sweden
Harm Reduction Journal (2023)
-
Integrated care model and point of care diagnostics facilitate Hepatitis C treatment among patients receiving opioid agonist therapy: a retrospective review of medical records
Substance Abuse Treatment, Prevention, and Policy (2022)
-
Hepatitis C reinfection in former and active injecting drug users in Belgium
Harm Reduction Journal (2021)
-
Opportunistic treatment of hepatitis C virus infection (OPPORTUNI-C): study protocol for a pragmatic stepped wedge cluster randomized trial of immediate versus outpatient treatment initiation among hospitalized people who inject drugs
Trials (2020)