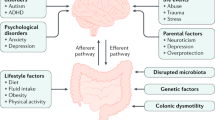
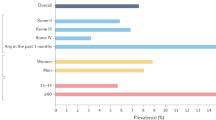
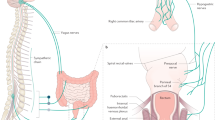
Key Points
-
Constipation is common, impairs quality of life and consumes considerable health-care resources
-
Recognition of whether constipation is primary or secondary is key for appropriate management
-
A detailed history and physical examination including digital rectal examination is important and can identify an evacuation disorder
-
Physiological tests such as colonic transit assessment, anorectal manometry and the balloon expulsion test can facilitate stratification of patients with different constipation subtypes
-
Newer drugs, such as linaclotide and lubiprostone, laxatives, and biofeedback therapy can considerably improve symptoms in patients with chronic constipation
Abstract
Constipation is a heterogeneous, polysymptomatic, multifactorial disease. Acute or transient constipation can be due to changes in diet, travel or stress, and secondary constipation can result from drug treatment, neurological or metabolic conditions or, rarely, colon cancer. A diagnosis of primary chronic constipation is made after exclusion of secondary causes of constipation and encompasses several overlapping subtypes. Slow-transit constipation is characterized by prolonged colonic transit in the absence of pelvic floor dysfunction. This subtype of constipation can be identified using either the radio-opaque marker test or wireless motility capsule test, and is best treated with laxatives such as polyethylene glycol or newer agents such as linaclotide or lubiprostone. If unsuccessful, subspecialist referral should be considered. Dyssynergic defecation results from impaired coordination of rectoanal and pelvic floor muscles, and causes difficulty with defecation. The condition can be identified using anorectal manometry and balloon expulsion tests and is best managed with biofeedback therapy. Opioid-induced constipation is an emerging entity, and several drugs including naloxegol, methylnaltrexone and lubiprostone are approved for its treatment. In this Review, we provide an overview of the burden and pathophysiology of chronic constipation, as well as a detailed discussion of the available diagnostic tools and treatment options.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Higgins, P. D. & Johanson, J. F. Epidemiology of constipation in North America: a systematic review. Am. J. Gastroenterol. 99, 750–759 (2004).
Belsey, J., Greenfield, S., Candy, D. & Geraint, M. Systematic review: impact of constipation on quality of life in adults and children. Aliment. Pharmacol. Ther. 31, 938–949 (2010).
Dennison, C. et al. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 23, 461–476 (2005).
Rao, S. S. et al. Psychological profiles and quality of life differ between patients with dyssynergia and those with slow transit constipation. J. Psychosom. Res. 63, 441–449 (2007).
Rao, S. S. Constipation: evaluation and treatment of colonic and anorectal motility disorders. Gastroenterol. Clin. North Am. 36, 687–711 (2007).
Pare, P., Ferrazzi, S., Thompson, W. G., Irvine, E. J. & Rance, L. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. Am. J. Gastroenterol. 96, 3130–3137 (2001).
Bharucha, A. E., Pemberton, J. H. & Locke, G. R. 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology 144, 218–238 (2013).
Mugie, S. M., Benninga, M. A. & Di Lorenzo, C. Epidemiology of constipation in children and adults: a systematic review. Best Pract. Res. Clin. Gastroenterol. 25, 3–18 (2011).
Dukas, L., Willett, W. C. & Giovannucci, E. L. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am. J. Gastroenterol. 98, 1790–1796 (2003).
Coremans, G., Margaritis, V. & Gebruers, K. Is there a genetic component in chronic functional constipation? Gastroenterology 128 (Suppl. 2), A272 (2005).
Mudipalli, R. S., Remes-Troche, J. M., Andersen, L. & Rao, S. S. Functional chest pain: esophageal or overlapping functional disorder. J. Clin. Gastroenterol. 41, 264–269 (2007).
Locke, G. R. et al. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol. Motil. 17, 29–34 (2005).
Rao, S. S. in Yamada's Textbook of Gastroenterology 6th edn (eds Podolsky, D. K. & Kalman, D.) 757–780 (John Wiley & Sons, 2016).
Burgell, R. E. et al. Assessment of rectal afferent neuronal function and brain activity in patients with constipation and rectal hyposensitivity. Neurogastroenterol. Motil. 25, 260–268 (2013).
Drossman, D. A. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 130, 1377–1390 (2006).
Rao, S. S., Sadeghi, P., Beaty, J. & Kavlock, R. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am. J. Gastroenterol. 99, 2405–2416 (2004).
Bassotti, G., de Roberto, G., Castellani, D., Sediari, L. & Morelli, A. Normal aspects of colorectal motility and abnormalities in slow transit constipation. World J. Gastroenterol. 11, 2691–2696 (2005).
Dinning, P. G. & Di Lorenzo, C. Colonic dysmotility in constipation. Best Pract. Res. Clin. Gastroenterol. 25, 89–101 (2011).
Dinning, P. G. et al. Pancolonic spatiotemporal mapping reveals regional deficiencies in, and disorganization of colonic propagating pressure waves in severe constipation. Neurogastroenterol. Motil. 22, e340–e349 (2010).
Rao, S. S., Sadeghi, P., Batterson, K. & Beaty, J. Altered periodic rectal motor activity: a mechanism for slow transit constipation. Neurogastroenterol. Motil. 13, 591–598 (2001).
Ravi, K. et al. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology 138, 89–97 (2010).
Bassotti, G., Iantorno, G., Fiorella, S., Bustos-Fernandez, L. & Bilder, C. R. Colonic motility in man: features in normal subjects and in patients with chronic idiopathic constipation. Am. J. Gastroenterol. 94, 1760–1770 (1999).
Herve, S. et al. Results of 24-h manometric recording of colonic motor activity with endoluminal instillation of bisacodyl in patients with severe chronic slow transit constipation. Neurogastroenterol. Motil. 16, 397–402 (2004).
Knowles, C. H. & Farrugia, G. Gastrointestinal neuromuscular pathology in chronic constipation. Best Pract. Res. Clin. Gastroenterol. 25, 43–57 (2011).
Rao, S. S., Tuteja, A. K., Vellema, T., Kempf, J. & Stessman, M. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J. Clin. Gastroenterol. 38, 680–685 (2004).
Rao, S. S., Welcher, K. D. & Leistikow, J. S. Obstructive defecation: a failure of rectoanal coordination. Am. J. Gastroenterol. 93, 1042–1050 (1998).
Reiner, C. S. et al. MR defecography in patients with dyssynergic defecation: spectrum of imaging findings and diagnostic value. Br. J. Radiol. 84, 136–144 (2011).
Rao, S. S., Ozturk, R., De Ocampo, S. & Stessman, M. Pathophysiology and role of biofeedback therapy in solitary rectal ulcer syndrome. Am. J. Gastroenterol. 101, 613–618 (2006).
Scott, S. M., van den Berg, M. M. & Benninga, M. A. Rectal sensorimotor dysfunction in constipation. Best Pract. Res. Clin. Gastroenterol. 25, 103–118 (2011).
Rattanakovit, K., Rao, S., Amieva, M., Mack, A. & Dewitt, A. Dyssynergia or no dyssynergia: phenotypic categorization of constipated patients with high resolution anorectal manometry (HRAM). Am. J. Gastroenterol. 110 (Suppl. 1s), S593 (2015).
Rao, S. S. Dyssynergic defecation and biofeedback therapy. Gastroenterol. Clin. North Am. 37, 569–586 (2008).
Chey, W. D., Kurlander, J. & Eswaran, S. Irritable bowel syndrome: a clinical review. JAMA 313, 949–958 (2015).
Spiller, R. & Lam, C. An update on post-infectious irritable bowel syndrome: role of genetics, immune activation, serotonin and altered microbiome. J. Neurogastroenterol. Motil. 18, 258–268 (2012).
Sachdev, A. H. & Pimentel, M. Antibiotics for irritable bowel syndrome: rationale and current evidence. Curr. Gastroenterol. Rep. 14, 439–445 (2012).
Shepherd, S. J., Lomer, M. C. & Gibson, P. R. Short-chain carbohydrates and functional gastrointestinal disorders. Am. J. Gastroenterol. 108, 707–717 (2013).
Halmos, E. P., Power, V. A., Shepherd, S. J., Gibson, P. R. & Muir, J. G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 146, 67–75 (2014).
Coss-Adame, E. & Rao, S. S. Brain and gut interactions in irritable bowel syndrome: new paradigms and new understandings. Curr. Gastroenterol. Rep. 16, 37 (2014).
Mayer, E. A. & Tillisch, K. The brain-gut axis in abdominal pain syndromes. Annu. Rev. Med. 62, 381–396 (2011).
Tantiphlachiva, K., Rao, P., Attaluri, A. & Rao, S. S. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin. Gastroenterol. Hepatol 8, 955–960 (2010).
Tornblom, H. et al. Colonic transit time and IBS symptoms: what's the link? Am. J. Gastroenterol. 107, 754–760 (2012).
Remes-Troche, J. M. & Rao, S. S. Diagnostic testing in patients with chronic constipation. Curr. Gastroenterol. Rep. 8, 416–424 (2006).
Videlock, E. J., Lembo, A. & Cremonini, F. Diagnostic testing for dyssynergic defecation in chronic constipation: meta-analysis. Neurogastroenterol. Motil. 25, 509–520 (2013).
Bertschinger, K. M. et al. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology 223, 501–508 (2002).
Dvorkin, L. S. et al. Open-magnet MR defaecography compared with evacuation proctography in the diagnosis and management of patients with rectal intussusception. Colorectal Dis. 6, 45–53 (2004).
Rao, S. S., Kavlock, R. & Rao, S. Influence of body position and stool characteristics on defecation in humans. Am. J. Gastroenterol. 101, 2790–2796 (2006).
Lee, Y. Y., Erdogan, A. & Rao, S. S. High resolution and high definition anorectal manometry and pressure topography: diagnostic advance or a new kid on the block? Curr. Gastroenterol. Rep. 15, 360 (2013).
Raizada, V., Bhargava, V., Karsten, A. & Mittal, R. K. Functional morphology of anal sphincter complex unveiled by high definition anal manometery and three dimensional ultrasound imaging. Neurogastroenterol. Motil. 23, 1013–e460 (2011).
Chiarioni, G., Kim, S. M., Vantini, I. & Whitehead, W. E. Validation of the balloon evacuation test: reproducibility and agreement with findings from anorectal manometry and electromyography. Clin. Gastroenterol. Hepatol. 12, 2049–2054 (2014).
Rao, S. S., Ozturk, R. & Laine, L. Clinical utility of diagnostic tests for constipation in adults: a systematic review. Am. J. Gastroenterol. 100, 1605–1615 (2005).
Camilleri, M. et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol. Motil. 22, 874–e233 (2010).
Rao, S. S. et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol. Motil. 23, 8–23 (2011).
Camilleri, M. et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol. Motil. 20, 1269–1282 (2008).
Singh, S., Heady, S., Coss-Adame, E. & Rao, S. S. Clinical utility of colonic manometry in slow transit constipation. Neurogastroenterol. Motil. 25, 487–495 (2013).
Markland, A. D. et al. Association of low dietary intake of fiber and liquids with constipation: evidence from the National Health and Nutrition Examination Survey. Am. J. Gastroenterol. 108, 796–803 (2013).
Rao, S. S., Yu, S. & Fedewa, A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment. Pharmacol. Ther. 41, 1256–1270 (2015).
Suares, N. C. & Ford, A. C. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment. Pharmacol. Ther. 33, 895–901 (2011).
Bijkerk, C. J., Muris, J. W., Knottnerus, J. A., Hoes, A. W. & de Wit, N. J. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 19, 245–251 (2004).
Attaluri, A., Donahoe, R., Valestin, J., Brown, K. & Rao, S. S. Randomised clinical trial: dried plums (prunes) versus psyllium for constipation. Aliment. Pharmacol. Ther. 33, 822–828 (2011).
Kamm, M. A. et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin. Gastroenterol. Hepatol 9, 577–583 (2011).
Chang, L., Lembo, A. & Sultan, S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology 147, 1149–1172 (2014).
Ford, A. C. & Suares, N. C. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut 60, 209–218 (2011).
Lee-Robichaud, H., Thomas, K., Morgan, J. & Nelson, R. L. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst. Rev. 7, CD007570 (2010).
Singh, S. & Rao, S. S. Pharmacologic management of chronic constipation. Gastroenterol. Clin. North Am. 39, 509–527 (2010).
Camilleri, M. et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G942–G947 (2006).
Lembo, A. J. et al. Long-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipation. Dig. Dis. Sci. 56, 2639–2645 (2011).
Busby, R. W. et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur. J. Pharmacol. 649, 328–335 (2010).
Castro, J. et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology 145, 1334–1346 (2013).
Chey, W. D. et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am. J. Gastroenterol. 107, 1702–1712 (2012).
Rao, S. et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am. J. Gastroenterol. 107, 1714–1724 (2012).
Camilleri, M. Guanylate cyclase C agonists: emerging gastrointestinal therapies and actions. Gastroenterology 148, 483–487 (2015).
Miner, P. B. et al. Plecanatide, a novel uroguanylin analog: a 12-week, randomized, double-blind, placebo-controlled, dose ranging trial to evaluate efficacy and safety in patients with irritable bowel syndrome with constipation (IBS-C). Am. J. Gastroenterol. 109 (Suppl. 2), S541 (2014).
Jarmuz, A., Zielinska, M., Storr, M. & Fichna, J. Emerging treatments in neurogastroenterology: perspectives of guanylyl cyclase C agonists use in functional gastrointestinal disorders and inflammatory bowel diseases. Neurogastroenterol. Motil. 27, 1057–1068 (2015).
Cash, B. D. & Chey, W. D. Review article: the role of serotonergic agents in the treatment of patients with primary chronic constipation. Aliment. Pharmacol. Ther. 22, 1047–1060 (2005).
Tack, J. & Corsetti, M. Prucalopride: evaluation of the pharmacokinetics, pharmacodynamics, efficacy and safety in the treatment of chronic constipation. Expert Opin. Drug Metab. Toxicol. 8, 1327–1335 (2012).
Shin, A. et al. Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment. Pharmacol. Ther. 39, 239–253 (2014).
Camilleri, M. et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol. Motil. 26, 1386–1395 (2014).
Garnock-Jones, K. P. & McKeage, K. Methynaltrexone. Drugs 70, 919–928 (2010).
Yuan, C. S. et al. Tolerability, gut effects, and pharmacokinetics of methylnaltrexone following repeated intravenous administration in humans. J. Clin. Pharmacol. 45, 538–546 (2005).
Siemens, W., Gaertner, J. & Becker, G. Advances in pharmacotherapy for opioid-induced constipation — a systematic review. Expert Opin. Pharmacother. 16, 515–532 (2015).
Tan, E. K., Cornish, J., Darzi, A. W. & Tekkis, P. P. Meta-analysis: Alvimopan versus placebo in the treatment of post-operative ileus. Aliment. Pharmacol. Ther. 25, 47–57 (2007).
US Department of Health & Human Services. Safety, Enterag (alvimopan) capsules. US Food and Drug Administration [online], (2013).
Corsetti, M. & Tack, J. Naloxegol, a new drug for the treatment of opioid-induced constipation. Expert Opin. Pharmacother. 16, 399–406 (2015).
Chey, W. D. et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N. Engl. J. Med. 370, 2387–2396 (2014).
Rao, S. S. et al. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol. Motil. 27, 594–609 (2015).
Rao, S. S. Dyssynergic defecation. Gastroenterol. Clin. North Am. 30, 97–114 (2001).
Rao, S. S. Biofeedback therapy for constipation in adults. Best Pract. Res. Clin. Gastroenterol. 25, 159–166 (2011).
Chiarioni, G., Whitehead, W. E., Pezza, V., Morelli, A. & Bassotti, G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 130, 657–664 (2006).
Heymen, S. et al. Prospective, randomized trial comparing four biofeedback techniques for patients with constipation. Dis. Colon Rectum 42, 1388–1393 (1999).
Rao, S. S. et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin. Gastroenterol. Hepatol. 5, 331–338 (2007).
Hallan, R. I. et al. Treatment of anismus in intractable constipation with botulinum A toxin. Lancet 2, 714–717 (1988).
Podzemny, V., Pescatori, L. C. & Pescatori, M. Management of obstructed defecation. World J. Gastroenterol. 21, 1053–1060 (2015).
Lee, H. J. et al. Long-term efficacy of biofeedback therapy in patients with dyssynergic defecation: results of a median 44 months follow-up. Neurogastroenterol. Motil. 27, 787–795 (2015).
Rao, S. S., Enck, P. & Loening-Baucke, V. Biofeedback therapy for defecation disorders. Dig. Dis. 15 (Suppl. 1), 78–92 (1997).
Rao, S. S., Erdogan, A., Coss-Adame, E., Valestin, J. & Mattos, M. L. Rectal hyposensitivity: randomized controlled trial of barostat versus syringe-assisted sensory training. Gastroenterology 144 (Suppl. 1), S363 (2013).
Hassan, I. et al. Ileorectal anastomosis for slow transit constipation: long-term functional and quality of life results. J. Gastrointest. Surg. 10, 1330–1336 (2006).
Redmond, J. M. et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am. J. Gastroenterol. 90, 748–753 (1995).
Pemberton, J. H., Rath, D. M. & Ilstrup, D. M. Evaluation and surgical treatment of severe chronic constipation. Ann. Surg. 214, 403–411 (1991).
Wexner, S. D., Daniel, N. & Jagelman, D. G. Colectomy for constipation: physiologic investigation is the key to success. Dis. Colon Rectum 34, 851–856 (1991).
Knowles, C. H., Scott, M. & Lunniss, P. J. Outcome of colectomy for slow transit constipation. Ann. Surg. 230, 627–638 (1999).
Preston, D. M., Hawley, P. R., Lennard-Jones, J. E. & Todd, I. P. Results of colectomy for severe idiopathic constipation in women (Arbuthnot Lane's disease). Br. J. Surg. 71, 547–552 (1984).
Ho, Y. H. & Goh, H. S. The investigation of chronic constipation for surgical management. Singapore Med. J. 37, 291–294 (1996).
Lundin, E., Karlbom, U., Pahlman, L. & Graf, W. Outcome of segmental colonic resection for slow-transit constipation. Br. J. Surg. 89, 1270–1274 (2002).
Abdulla, H., Yu, S., Rattanakovit, K., Badger, C. & Rao, S. S. Small intestinal bacterial overgrowth (SIBO) & fungal overgrowth (SIFO): a frequent & unrecognized complication of colectomy. Am. J. Gastroenterol. 110 (Suppl. 1s), S995 (2015).
Lees, N. P., Hodson, P., Hill, J., Pearson, R. C. & Maclennan, I. Long-term results of the antegrade continent enema procedure for constipation in adults. Colorectal Dis. 6, 362–368 (2004).
Rivera, M. T., Kugathasan, S., Berger, W. & Werlin, S. L. Percutaneous colonoscopic cecostomy for management of chronic constipation in children. Gastrointest. Endosc. 53, 225–228 (2001).
Tackett, L. D., Minevich, E., Benedict, J. F., Wacksman, J. & Sheldon, C. A. Appendiceal versus ileal segment for antegrade continence enema. J. Urol. 167, 683–686 (2002).
Kamm, M. A. et al. Sacral nerve stimulation for intractable constipation. Gut 59, 333–340 (2010).
Thaha, M. A., Abukar, A. A., Thin, N. N., Ramsanahie, A. & Knowles, C. H. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst. Rev. 8, CD004464 (2015).
Dinning, P. G. et al. Treatment efficacy of sacral nerve stimulation in slow transit constipation: a two-phase, double-blind randomized controlled crossover study. Am. J. Gastroenterol. 110, 733–740 (2015).
Ramkumar, D. & Rao, S. S. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am. J. Gastroenterol. 100, 936–971 (2005).
Brandt, L. J. et al. Systematic review on the management of chronic constipation in North America. Am. J. Gastroenterol. 100 (Suppl. 1), S5–S21 (2005).
Acknowledgements
The authors would like to thank H. Smith (Medical College of Georgia, Augusta, Georgia, USA) for technical and secretarial assistance.
Author information
Authors and Affiliations
Contributions
K.R. drafted the article. K.R., S.S.C.R. and T. P. researched data for the article, provided a substantial contribution to discussion of the content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.S.C.R. serves on the advisory boards of Forest Labs, Salix, Synergy and Takeda, and has reviewed research grants from Forest Labs and Medtronic. K.R. and T.P. declare no competing interests.
Rights and permissions
About this article
Cite this article
Rao, S., Rattanakovit, K. & Patcharatrakul, T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol 13, 295–305 (2016). https://doi.org/10.1038/nrgastro.2016.53
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.53
This article is cited by
-
Physical and psychological correlates of somatic symptom in patients with functional constipation: a cross-sectional study
BMC Psychiatry (2024)
-
Lacidophilin tablets alleviate constipation through regulation of intestinal microflora by promoting the colonization of Akkermansia sps
Scientific Reports (2024)
-
Effects of natural products on functional constipation: analysis of active ingredient and mechanism
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Effectiveness and safety of digital rectal stimulation and abdominal massage for neurogenic bowel dysfunction in stroke patients: a randomized controlled trial protocol
Trials (2023)
-
Systemic sclerosis gastrointestinal dysmotility: risk factors, pathophysiology, diagnosis and management
Nature Reviews Rheumatology (2023)