Key Points
-
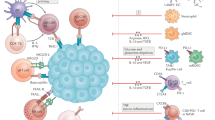
Hepatocellular carcinoma (HCC) is an immunogenic liver lesion that expresses shared tumour antigens (tumour-associated antigens) and private neo-antigens arising from specific gene mutations
-
Despite HCC antigenicity and intratumour accumulation of effector T cells, antitumour immune responses are subverted by a variety of stromal cells and multiple immunoinhibitory molecules
-
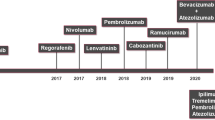
Different immunotherapeutic modalities have been used to treat HCC, including diverse vaccine platforms, adoptive T-cell therapy, cytokines, gene therapy and monoclonal antibodies that target immune checkpoint molecules
-
The abundance of additive immunosuppressive factors in the HCC microenvironment calls for a multitargeted approach, combining systemic and locoregional therapeutic modalities
-
Administration of monoclonal antibodies, adoptive T-cell therapy or vaccines in combination with gene therapy vectors that encode monoclonal antibodies and/or immunostimulatory cytokines are powerful strategies to treat HCC
Abstract
Advanced hepatocellular carcinoma (HCC) is a serious therapeutic challenge and targeted therapies only provide a modest benefit in terms of overall survival. Novel approaches are urgently needed for the treatment of this prevalent malignancy. Evidence demonstrating the antigenicity of tumour cells, the discovery that immune checkpoint molecules have an essential role in immune evasion of tumour cells, and the impressive clinical results achieved by blocking these inhibitory receptors, are revolutionizing cancer immunotherapy. Here, we review the data on HCC immunogenicity, the mechanisms for HCC immune subversion and the different immunotherapies that have been tested to treat HCC. Taking into account the multiplicity of hyperadditive immunosuppressive forces acting within the HCC microenvironment, a combinatorial approach is advised. Strategies include combinations of systemic immunomodulation and gene therapy, cell therapy or virotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
03 November 2015
In the version of this article originally published online an affiliation was missing for Bruno Sangro and has now been added. The error has been corrected for the print, HTML and PDF versions of the article.
References
European Association For The Study Of The Liver & European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 56, 908–943 (2012).
Sangro, B. et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 54, 868–878 (2011).
Llovet, J. M. & Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37, 429–442 (2003).
Giannini, E. G. et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 61, 184–190 (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
Flecken, T. et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 59, 1415–1426 (2014).
Hato, T., Goyal, L., Greten, T. F., Duda, D. G. & Zhu, A. X. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology 60, 1776–1782 (2014).
Greten, T. F. et al. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer 10, 209 (2010).
Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546–1558 (2013).
Kudo, M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J. Gastroenterol. 44, S112–S118 (2009).
Yoong, K. F., McNab, G., Hubscher, S. G. & Adams, D. H. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. J. Immunol. 160, 3978–3988 (1998).
Unitt, E. et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J. Hepatol. 45, 246–253 (2006).
Wada, Y., Nakashima, O., Kutami, R., Yamamoto, O. & Kojiro, M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 27, 407–414 (1998).
Willimsky, G., Schmidt, K., Loddenkemper, C., Gellermann, J. & Blankenstein, T. Virus-induced hepatocellular carcinomas cause antigen-specific local tolerance. J. Clin. Invest. 123, 1032–1043 (2013).
Anguille, S. et al. Dendritic cells as pharmacological tools for cancer immunotherapy. Pharmacol. Rev. 67, 731–753 (2015).
Mittal, D., Gubin, M. M., Schreiber, R. D. & Smyth, M. J. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).
Bhadra, R., Cobb, D. A. & Khan, I. A. CD40 signaling to the rescue: a CD8 exhaustion perspective in chronic infectious diseases. Crit. Rev. Immunol. 33, 361–378 (2013).
Coughlin, C. M. et al. Tumor cell responses to IFNγ affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity 9, 25–34 (1998).
Budhu, A. et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 10, 99–111 (2006).
Quezada, S. A., Peggs, K. S., Simpson, T. R. & Allison, J. P. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol. Rev. 241, 104–118 (2011).
Burzyn, D. et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 (2013).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Khong, H. T. & Restifo, N. P. Natural selection of tumor variants in the generation of 'tumor escape' phenotypes. Nat. Immunol. 3, 999–1005 (2002).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Champsaur, M. & Lanier, L. L. Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 235, 267–285 (2010).
Mondelli, M. U. NKG2D and its ligands: key to immunotherapy of liver cancer? J. Hepatol. 56, 308–310 (2012).
Jinushi, M. et al. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J. Hepatol. 43, 1013–1020 (2005).
Kohga, K. et al. Sorafenib inhibits the shedding of major histocompatibility complex class I-related chain A on hepatocellular carcinoma cells by down-regulating a disintegrin and metalloproteinase 9. Hepatology 51, 1264–1273 (2010).
Kamimura, H. et al. Reduced NKG2D ligand expression in hepatocellular carcinoma correlates with early recurrence. J. Hepatol. 56, 381–388 (2012).
Yan, W. et al. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut http://dx.doi.org/10.1136/gutjnl-2014-307671.
Yan, W. et al. Netrin-1 induces epithelial-mesenchymal transition and promotes hepatocellular carcinoma invasiveness. Dig. Dis. Sci. 59, 1213–1221 (2014).
Pan, K. et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 134, 1247–1253 (2008).
Zhang, Z., Zhang, Y., Sun, X. X., Ma, X. & Chen, Z. N. microRNA-146a inhibits cancer metastasis by downregulating VEGF through dual pathways in hepatocellular carcinoma. Mol. Cancer 14, 5 (2015).
Lu, Y., Lin, N., Chen, Z. & Xu, R. Hypoxia-induced secretion of platelet-derived growth factor-BB by hepatocellular carcinoma cells increases activated hepatic stellate cell proliferation, migration and expression of vascular endothelial growth factor-A. Mol. Med. Rep. 11, 691–697 (2015).
Castillo, J. et al. Amphiregulin induces the alternative splicing of p73 into its oncogenic isoform ΔEx2p73 in human hepatocellular tumors. Gastroenterology 137, 1805–1815.e4 (2009).
Chen, K. J. et al. Selective recruitment of regulatory T cell through CCR6–CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE 6, e24671 (2011).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Blay, J., White, T. D. & Hoskin, D. W. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 57, 2602–2605 (1997).
Gessi, S., Merighi, S., Sacchetto, V., Simioni, C. & Borea, P. A. Adenosine receptors and cancer. Biochim. Biophys. Acta 1808, 1400–1412 (2011).
Hoechst, B. et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology 135, 234–243 (2008).
Arihara, F. et al. Increase in CD14+HLA-DR−/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol. Immunother. 62, 1421–1430 (2013).
Ostrand-Rosenberg, S., Sinha, P., Beury, D. W. & Clements, V. K. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin. Cancer Biol. 22, 275–281 (2012).
Nagaraj, S. et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 13, 828–835 (2007).
Li, H., Han, Y., Guo, Q., Zhang, M. & Cao, X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β 1. J. Immunol. 182, 240–249 (2009).
Lin, E. Y. et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 66, 11238–11246 (2006).
Qian, B. et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 4, e6562 (2009).
Chen, Y. et al. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 α/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology 59, 1435–1447 (2014).
Orimo, A. & Weinberg, R. A. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5, 1597–1601 (2006).
Infante, J. R. et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 25, 319–325 (2007).
Li, T. et al. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 318, 154–161 (2012).
Neaud, V. et al. Human hepatic myofibroblasts increase invasiveness of hepatocellular carcinoma cells: evidence for a role of hepatocyte growth factor. Hepatology 26, 1458–1466 (1997).
Hochst, B. et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J. Hepatol. 59, 528–535 (2013).
Dunham, R. M. et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J. Immunol. 190, 2009–2016 (2013).
Perugorria, M. J. et al. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology 48, 1251–1261 (2008).
Zaiss, D. M. et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 38, 275–284 (2013).
Wu, S. D. et al. Role of the microenvironment in hepatocellular carcinoma development and progression. Cancer Treat. Rev. 38, 218–225 (2012).
Hernandez-Gea, V., Toffanin, S., Friedman, S. L. & Llovet, J. M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 144, 512–527 (2013).
Carambia, A. et al. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J. Hepatol. 61, 594–599 (2014).
Motz, G. T. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615 (2014).
Han, Y. et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 59, 567–579 (2014).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Quezada, S. A. & Peggs, K. S. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br. J. Cancer 108, 1560–1565 (2013).
Sprinzl, M. F. & Galle, P. R. Immune control in hepatocellular carcinoma development and progression: role of stromal cells. Semin. Liver Dis. 34, 376–388 (2014).
Berasain, C., Castillo, J., Perugorria, M. J., Prieto, J. & Avila, M. A. Amphiregulin: a new growth factor in hepatocarcinogenesis. Cancer Lett. 254, 30–41 (2007).
Zhou, J. et al. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int. J. Cancer 125, 1640–1648 (2009).
Moo-Young, T. A. et al. Tumor-derived TGF-β mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J. Immunother. 32, 12–21 (2009).
Nguyen, L. T. & Ohashi, P. S. Clinical blockade of PD1 and LAG3—potential mechanisms of action. Nat. Rev. Immunol. 15, 45–56 (2015).
Fourcade, J. et al. CD8+ T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 72, 887–896 (2012).
Krummel, M. F. & Allison, J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182, 459–465 (1995).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Peggs, K. S., Quezada, S. A., Chambers, C. A., Korman, A. J. & Allison, J. P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009).
Francisco, L. M. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 (2009).
Said, E. A. et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16, 452–459 (2010).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Latchman, Y. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2, 261–268 (2001).
Zou, W. & Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 8, 467–477 (2008).
Zabala, M. et al. Induction of immunosuppressive molecules and regulatory T cells counteracts the antitumor effect of interleukin-12-based gene therapy in a transgenic mouse model of liver cancer. J. Hepatol. 47, 807–815 (2007).
Noman, M. Z. et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 (2014).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Shi, F. et al. PD-1 and PD-L1 upregulation promotes CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer 128, 887–896 (2011).
Krempski, J. et al. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J. Immunol. 186, 6905–6913 (2011).
Gao, Q. et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin. Cancer Res. 15, 971–979 (2009).
Nakayama, M. et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113, 3821–3830 (2009).
Zhu, C. et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6, 1245–1252 (2005).
Wada, J., Ota, K., Kumar, A., Wallner, E. I. & Kanwar, Y. S. Developmental regulation, expression, and apoptotic potential of galectin-9, a β-galactoside binding lectin. J. Clin. Invest. 99, 2452–2461 (1997).
Monney, L. et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415, 536–541 (2002).
Gautron, A. S., Dominguez-Villar, M., de Marcken, M. & Hafler, D. A. Enhanced suppressor function of TIM-3+FoxP3+ regulatory T cells. Eur. J. Immunol. 44, 2703–2711 (2014).
Li, H. et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 56, 1342–1351 (2012).
Triebel, F. et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 171, 1393–1405 (1990).
Workman, C. J., Rice, D. S., Dugger, K. J., Kurschner, C. & Vignali, D. A. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3). Eur. J. Immunol. 32, 2255–2263 (2002).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Woo, S. R. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012).
Grosso, J. F. et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 117, 3383–3392 (2007).
Matsuzaki, J. et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl Acad. Sci. USA 107, 7875–7880 (2010).
Pasero, C. & Olive, D. Interfering with coinhibitory molecules: BTLA/HVEM as new targets to enhance anti-tumor immunity. Immunol. Lett. 151, 71–75 (2013).
Hokuto, D. et al. Clinical impact of herpesvirus entry mediator expression in human hepatocellular carcinoma. Eur. J. Cancer 51, 157–165 (2015).
Mezrich, J. D. et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185, 3190–3198 (2010).
Belladonna, M. L., Orabona, C., Grohmann, U. & Puccetti, P. TGF-β and kynurenines as the key to infectious tolerance. Trends Mol. Med. 15, 41–49 (2009).
Mellor, A. L. & Munn, D. H. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat. Rev. Immunol. 8, 74–80 (2008).
Zhao, Q. et al. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J. Immunol. 188, 1117–1124 (2012).
Mao, Y., Poschke, I. & Kiessling, R. Tumour-induced immune suppression: role of inflammatory mediators released by myelomonocytic cells. J. Intern. Med. 276, 154–170 (2014).
Montesinos, M. C. et al. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A2A receptors. Am. J. Pathol. 160, 2009–2018 (2002).
Zarek, P. E. et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111, 251–259 (2008).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Liu, F. T. & Rabinovich, G. A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29–41 (2005).
Cedeno-Laurent, F. & Dimitroff, C. J. Galectins and their ligands: negative regulators of anti-tumor immunity. Glycoconj. J. 29, 619–625 (2012).
Matsuda, Y., Yamagiwa, Y., Fukushima, K., Ueno, Y. & Shimosegawa, T. Expression of galectin-3 involved in prognosis of patients with hepatocellular carcinoma. Hepatol. Res. 38, 1098–1111 (2008).
Cedeno-Laurent, F., Opperman, M., Barthel, S. R., Kuchroo, V. K. & Dimitroff, C. J. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. J. Immunol. 188, 3127–3137 (2012).
Peng, W., Wang, H. Y., Miyahara, Y., Peng, G. & Wang, R. F. Tumor-associated galectin-3 modulates the function of tumor-reactive T cells. Cancer Res. 68, 7228–7236 (2008).
Kouo, T. et al. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol. Res. 3, 412–23 (2015).
Sprinzl, M. F. et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology 57, 2358–2368 (2013).
Akiba, J., Yano, H., Ogasawara, S., Higaki, K. & Kojiro, M. Expression and function of interleukin-8 in human hepatocellular carcinoma. Int. J. Oncol. 18, 257–264 (2001).
Fernando, R. I., Castillo, M. D., Litzinger, M., Hamilton, D. H. & Palena, C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 71, 5296–5306 (2011).
Llovet, J. M. et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 18, 2290–2300 (2012).
Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212, 139–148 (2015).
Philips, G. K. & Atkins, M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int. Immunol. 27, 39–46 (2015).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Yadav, M. et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515, 572–576 (2014).
Sangro, B. & Prieto, J. Gene therapy for liver cancer: clinical experience and future prospects. Curr. Opin. Mol. Ther. 12, 561–569 (2010).
Therasse, P., Eisenhauer, E. A. & Verweij, J. RECIST revisited: a review of validation studies on tumour assessment. Eur. J. Cancer 42, 1031–1039 (2006).
Schlom, J. Therapeutic cancer vaccines: current status and moving forward. J. Natl Cancer Inst. 104, 599–613 (2012).
Hoos, A. et al. Improved endpoints for cancer immunotherapy trials. J. Natl Cancer Inst. 102, 1388–1397 (2010).
Llovet, J. M. et al. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology 31, 54–58 (2000).
Chen, L. T. et al. Long-term results of a randomized, observation-controlled, Phase III trial of adjuvant interferon α-2b in hepatocellular carcinoma after curative resection. Ann. Surg. 255, 8–17 (2012).
Boisguerin, V. et al. Translation of genomics-guided RNA-based personalised cancer vaccines: towards the bedside. Br. J. Cancer 111, 1469–1475 (2014).
Sawada, Y. et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin. Cancer Res. 18, 3686–3696 (2012).
Radford, K. J., Tullett, K. M. & Lahoud, M. H. Dendritic cells and cancer immunotherapy. Curr. Opin. Immunol. 27, 26–32 (2014).
Quinn, D. I., Shore, N. D., Egawa, S., Gerritsen, W. R. & Fizazi, K. Immunotherapy for castration-resistant prostate cancer: progress and new paradigms. Urol. Oncol. 33, 245–260 (2015).
Tullett, K. M., Lahoud, M. H. & Radford, K. J. Harnessing human cross-presenting CLEC9A+XCR1+ dendritic cells for immunotherapy. Front. Immunol. 5, 239 (2014).
Jongbloed, S. L. et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 207, 1247–1260 (2010).
Bachem, A. et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 207, 1273–1281 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Tada, F. et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 41, 1601–1609 (2012).
Butterfield, L. H. et al. A Phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four α-fetoprotein peptides. Clin. Cancer Res. 12, 2817–2825 (2006).
El Ansary, M. et al. Immunotherapy by autologous dendritic cell vaccine in patients with advanced HCC. J. Cancer Res. Clin. Oncol. 139, 39–48 (2013).
Palmer, D. H. et al. A Phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 49, 124–132 (2009).
Nakamoto, Y. et al. Combined therapy of transcatheter hepatic arterial embolization with intratumoral dendritic cell infusion for hepatocellular carcinoma: clinical safety. Clin. Exp. Immunol. 147, 296–305 (2007).
Qiu, Y. et al. Hepatocellular carcinoma-specific immunotherapy with synthesized α1,3- galactosyl epitope-pulsed dendritic cells and cytokine-induced killer cells. World J. Gastroenterol. 17, 5260–5266 (2011).
Lee, W. C. et al. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J. Immunother. 28, 496–504 (2005).
Mazzolini, G. et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J. Clin. Oncol. 23, 999–1010 (2005).
Chi, K. H. et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J. Immunother. 28, 129–135 (2005).
Mazzolini, G. et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J. Clin. Oncol. 23, 999–1010 (2005).
Morisaki, T. et al. NKG2D-directed cytokine-activated killer lymphocyte therapy combined with gemcitabine for patients with chemoresistant metastatic solid tumors. Anticancer Res. 34, 4529–4538 (2014).
Phan, G. Q. & Rosenberg, S. A. Adoptive cell transfer for patients with metastatic melanoma: the potential and promise of cancer immunotherapy. Cancer Control 20, 289–297 (2013).
Besser, M. J. et al. Clinical responses in a Phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 16, 2646–2655 (2010).
Shi, H., Liu, L. & Wang, Z. Improving the efficacy and safety of engineered T cell therapy for cancer. Cancer Lett. 328, 191–197 (2013).
Johnson, L. A. et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009).
Parkhurst, M. R. et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 19, 620–626 (2011).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
Jensen, M. C. & Riddell, S. R. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol. Rev. 257, 127–144 (2014).
Till, B. G. et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950 (2012).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Shi, M. et al. Autologous cytokine-induced killer cell therapy in clinical trial Phase I is safe in patients with primary hepatocellular carcinoma. World J. Gastroenterol. 10, 1146–1151 (2004).
Ma, H. et al. Therapeutic safety and effects of adjuvant autologous RetroNectin activated killer cell immunotherapy for patients with primary hepatocellular carcinoma after radiofrequency ablation. Cancer. Biol. Ther. 9, 903–907 (2010).
Takayama, T. et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 356, 802–807 (2000).
Hui, D., Qiang, L., Jian, W., Ti, Z. & Da-Lu, K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig. Liver Dis. 41, 36–41 (2009).
Pan, K. et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann. Surg. Oncol. 20, 4305–4311 (2013).
Weng, D. S. et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J. Immunother. 31, 63–71 (2008).
Huang, Z. M. et al. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J. Immunother. 36, 287–293 (2013).
Yu, X. et al. A randomized Phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J. Clin. Immunol. 34, 194–203 (2014).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014).
Rammensee, H. G. & Singh-Jasuja, H. HLA ligandome tumor antigen discovery for personalized vaccine approach. Expert Rev. Vaccines 12, 1211–1217 (2013).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Furness, A. J., Vargas, F. A., Peggs, K. S. & Quezada, S. A. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol. 35, 290–298 (2014).
Sangro, B. et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 59, 81–88 (2013).
El-Khoueiry, A. B. et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209–040. J. Clin. Oncol. 33, LBA101 (2015).
Segal, N. H. et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J. Clin. Oncol. 32, S3002 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Filmus, J. & Selleck, S. B. Glypicans: proteoglycans with a surprise. J. Clin. Invest. 108, 497–501 (2001).
Zhu, A. X. et al. First-in-man Phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 19, 920–928 (2013).
Feng, M. & Ho, M. Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett. 588, 377–382 (2014).
Hoffmann-La Roche. A randomised, placebo-controlled, double-blind, multicenter Phase II trial of intravenous GC33 at 1600 mg Q2W in previously treated patients with unresectable advanced or metastatic hepatocellular carcinoma (HCC). ClinicalTrials.gov [online], (2014).
Melero, I., Hirschhorn-Cymerman, D., Morales-Kastresana, A., Sanmamed, M. F. & Wolchok, J. D. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin. Cancer Res. 19, 1044–1053 (2013).
Beatty, G. L. et al. A Phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin. Cancer Res. 19, 6286–6295 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Gonzalez-Aseguinolaza, G. & Prieto, J. Gene therapy of liver diseases: a 2011 perspective. Clin. Res. Hepatol. Gastroenterol. 35, 699–708 (2011).
Hernández-Alcoceba, R., Sangro, B., Berraondo, P., Gonzalez-Aseguinolaza, G. & Prieto, J. Cytokines for the treatment of gastrointestinal cancers: clinical experience and new perspectives. Expert Opin. Investig. Drugs 22, 827–841 (2013).
Hernández-Alcoceba, R. Recent advances in oncolytic virus design. Clin. Transl. Oncol. 13, 229–239 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Quetglas, J. I. et al. Virotherapy with a Semliki Forest virus-based vector encoding IL-12 synergizes with PD-1/PD-L1 blockade. Cancer Immunol. Res. 3, 449–454 (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2008).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Suscovich, T. J. & Alter, G. In situ production of therapeutic monoclonal antibodies. Expert Rev. Vaccines 14, 205–219 (2015).
Sangro, B. et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J. Clin. Oncol. 22, 1389–1397 (2004).
Park, B. H. et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a Phase I trial. Lancet Oncol. 9, 533–542 (2008).
Heo, J. et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 19, 329–336 (2013).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Gros, A. et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Wang, L. et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J. Clin. Invest. 121, 2371–2382 (2011).
Allard, B., Pommey, S., Smyth, M. J. & Stagg, J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin. Cancer Res. 19, 5626–5635 (2013).
Sawada, Y. et al. Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int. J. Oncol. 46, 28–36 (2015).
Le, D. T. et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. 36, 382–389 (2013).
Karyampudi, L. et al. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 74, 2974–2985 (2014).
Hirschhorn-Cymerman, D. et al. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J. Exp. Med. 209, 2113–2126 (2012).
Hernandez-Chacon, J. A. et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J. Immunother. 34, 236–250 (2011).
Engeland, C. E. et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 22, 1949–1959 (2014).
Quetglas, J. I. et al. Immunotherapeutic synergy between anti-CD137 mAb and intratumoral administration of a cytopathic Semliki Forest virus encoding IL-12. Mol. Ther. 20, 1664–1675 (2012).
Zerbini, A. et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 66, 1139–1146 (2006).
Victor, C. T. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 16, 373–377 (2015).
Duffy, A. G. et al. A pilot study of tremelimumab—a monoclonal antibody against CTLA-4–in combination with either trans catheter arterial chemoembolization (TACE) or radiofrequency ablation (RFA) in patients with hepatocellular carcinoma (HCC). J. Clin. Oncol. 33, S4081 (2015).
Duffy, A. G. & Greten, T. F. Immunological off-target effects of standard treatments in gastrointestinal cancers. Ann. Oncol. 25, 24–32 (2014).
Gonzalez-Aparicio, M. et al. Self-inactivating helper virus for the production of high-capacity adenoviral vectors. Gene Ther. 18, 1025–1033 (2011).
Kawaguchi, Y. et al. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int. J. Cancer 120, 781–787 (2007).
Desar, I. M. et al. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. Int. J. Cancer 129, 507–512 (2011).
Zhu, X. D. et al. Antiangiogenic therapy promoted metastasis of hepatocellular carcinoma by suppressing host-derived interleukin-12b in mouse models. Angiogenesis 16, 809–820 (2013).
Hipp, M. M. et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 111, 5610–5620 (2008).
Wang, W. et al. Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J. Transl. Med. 10, 146 (2012).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Postow, M. A. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017 (2015).
Le, D. T. et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Acknowledgements
The authors wish to express deep gratitude to colleagues of the Division of Hepatology and Gene Therapy of the Centre of Applied Medical Research (CIMA) of the University of Navarra, the Liver Unit and the Clinical Trial Unit of the University Clinic of Navarra, Drs J.J. Lasarte, P. Sarobe, M. Avila, F. Corrales, C. Berasain, R. Hernandez-Alcoceba, C. Smerdou, P. Berraondo, R. Aldabe, G. Gonzalez-Aseguinolaza, J. Quiroga, M. Iñarrairaegui, J.I. Herrero, J.L. Perez-Gracia as well as to Dr Ch. Qian (Chongching Military Hospital) for their collaboration, inspiration and input in research and medical assistance during many years as well as the secretary M.E. Perez-Mena for her help in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
J.P. and B.S. contributed equally to researching data for the manuscript, discussion of content and writing. All authors contributed equally to reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
B.S. has acted as a consultant to Bristol–Myers Squibb. I.M. has acted as a consultant to AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Genentech, LeadArtis, Miltenyi Biotec, Roche. J.P. declares no competing interests.
Supplementary information
Supplementary Table 1
Immunotherapies with FDA-approval for cancer treatment (DOC 84 kb)
Supplementary Table 2
Clinical trials on cell-based immunotherapies (DCs) in HCC (DOC 84 kb)
Supplementary Table 3
Clinical trials on cell-based immunotherapies (CIKs) in HCC (DOC 84 kb)
Rights and permissions
About this article
Cite this article
Prieto, J., Melero, I. & Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 12, 681–700 (2015). https://doi.org/10.1038/nrgastro.2015.173
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.173
This article is cited by
-
Immune checkpoint inhibitors in gastrointestinal malignancies: an Umbrella review
Cancer Cell International (2024)
-
Targeting ALK averts ribonuclease 1-induced immunosuppression and enhances antitumor immunity in hepatocellular carcinoma
Nature Communications (2024)
-
Carnosic acid nanocluster-based framework combined with PD-1 inhibitors impeded tumorigenesis and enhanced immunotherapy in hepatocellular carcinoma
Functional & Integrative Genomics (2024)
-
The efficacy and safety of PD-1 inhibitor combined with TACE in the first-line treatment of unresectable hepatocellular carcinoma
Medical Oncology (2024)
-
Characterization and clinical verification of immune-related genes in hepatocellular carcinoma to aid prognosis evaluation and immunotherapy
BMC Cancer (2023)