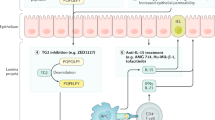
Key Points
-
The most common cause of persistent villous atrophy is inadvertent gluten exposure, but refractory coeliac disease can occur
-
Autoimmune enteropathy, common variable immune deficiency and olmesartan-related enteropathy should be excluded in instances of persistent villous atrophy
-
Type II refractory coeliac disease is now considered a low-grade, no-mass lymphoma
-
Patients with enteropathy-associated T-cell lymphoma and refractory coeliac disease should be treated in dedicated coeliac and small bowel disease centres
Abstract
A small subset of patients with coeliac disease become refractory to a gluten-free diet with persistent malabsorption and intestinal villous atrophy. The most common cause of this condition is inadvertent gluten exposure, but concomitant diseases leading to villous atrophy should also be considered and excluded. After exclusion of these conditions, patients are referred to as having refractory coeliac disease, of which two categories are recognized based on the absence (type I) or presence (type II) of a clonal expansion of premalignant intraepithelial lymphocyte population with a high potential for transformation into an overt enteropathy-associated T-cell lymphoma. Type I disease usually has a benign course that can be controlled by mild immunosuppressive treatment, but type II can be more severe with cladribine with or without autologous stem cell transplantation effective as treatment. Patients who fail to respond to cladribine therapy, however, still have a high risk of malignant transformation. Insights into the immunophenotype of these cells and the recognition that type II disease is a low-grade, no-mass lymphoma opens avenues for new treatment strategies, including chemotherapeutic and immunomodulating strategies. This Review will provide an overview of refractory coeliac disease, discussing mechanisms, diagnosis and management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
16 September 2015
In the version of this article originally published online, HLA-DQ2 and fludarabine were misspelled in Figure 1. These errors have been corrected for the PDF, HTML and print versions of the article.
References
Castillo, N. E., Theethira, T. G. & Leffler, D. A. The present and the future in the diagnosis and management of celiac disease. Gastroenterol. Rep. (Oxf.) 3, 3–11 (2014).
Al-Toma, A., Verbeek, W. H. & Mulder, C. J. Update on the management of refractory coeliac disease. J. Gastrointest. Liver Dis. 16, 57–63 (2007).
Wahab, P. J., Meijer, J. W. & Mulder, C. J. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am. J. Clin. Pathol. 118, 459–463 (2002).
Lanzini, A. et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 29, 1299–1308 (2009).
Kaukinen, K. et al. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment. Pharmacol. Ther. 25, 1237–1245 (2007).
United European Gastroenterology. When is a coeliac a coeliac? Report of a working group of the United European Gastroenterology Week in Amsterdam. Eur. J. Gastroenterol. Hepatol. 13, 1123–1128 (2001).
Al-Toma, A., Verbeek, W. H., Hadithi, M, von Blomberg, B. M. & Mulder, C. J. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut 56, 1373–1378 (2007).
Rubio-Tapia, A. et al. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology 136, 99–107 (2009).
Malamut, G. et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology 136, 81–90 (2009).
West, J. Celiac disease and its complications: a time traveller's perspective. Gastroenterology 136, 32–34 (2009).
Roshan, B. et al. The incidence and clinical spectrum of refractory celiac disease in a North American referral center. Am. J. Gastroenterol. 106, 923–928 (2011).
Wolters, V. M. & Wijmenga, C. Genetic background of celiac disease and its clinical implications. Am. J. Gastroenterol. 103, 190–195 (2008).
Al-Toma, A. et al. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathy-associated T-cell lymphoma. Clin. Gastroenterol. Hepatol. 4, 315–319 (2006).
Di, S. A., Biagi, F., Gobbi, P. G. & Corazza, G. R. How I treat enteropathy-associated T-cell lymphoma. Blood 119, 2458–2468 (2012).
Tack, G. J., Verbeek, W. H., Schreurs, M. W. & Mulder, C. J. The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat. Rev. Gastroenterol. Hepatol. 7, 204–213 (2010).
Malamut, G., Meresse, B., Cellier, C. & Cerf-Bensussan, N. Refractory celiac disease: from bench to bedside. Semin. Immunopathol. 34, 601–613 (2012).
Verbeek, W. H. et al. Flow cytometric determination of aberrant intra-epithelial lymphocytes predicts T-cell lymphoma development more accurately than T-cell clonality analysis in refractory celiac disease. Clin. Immunol. 126, 48–56 (2008).
Tack, G. J. et al. Serum parameters in the spectrum of coeliac disease: beyond standard antibody testing—a cohort study. BMC Gastroenterol. 12, 159 (2012).
Cellier, C. et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology 114, 471–481 (1998).
Schmitz, F. et al. Identification of a potential physiological precursor of aberrant cells in refractory coeliac disease type II. Gut 62, 509–519 (2013).
Mention, J. J. et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125, 730–745 (2003).
Colpitts, S. L. et al. Cutting edge: the role of IFN-alpha receptor and MyD88 signaling in induction of IL-15 expression in vivo. J. Immunol. 188, 2483–2487 (2012).
Rubio-Tapia, A. & Murray, J. A. Classification and management of refractory coeliac disease. Gut 59, 547–557 (2010).
Al-Toma, A., Verbeek, W. H. & Mulder, C. J. The management of complicated celiac disease. Dig. Dis. 25, 230–236 (2007).
Daum, S., Cellier, C. & Mulder, C. J. Refractory coeliac disease. Best Pract. Res. Clin. Gastroenterol. 19, 413–424 (2005).
Hadithi M et al. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann. Intern. Med. 147, 294–302 (2007).
Vahedi, K. et al. Reliability of antitransglutaminase antibodies as predictors of gluten-free diet compliance in adult celiac disease. Am. J. Gastroenterol. 98, 1079–1087 (2003).
[No authors listed]. Giardia lamblia and coeliac disease. Lancet 302, 138 (1973).
Mulder, C. J., Wahab, P. J., Moshaver, B. & Meijer, J. W. Refractory coeliac disease: a window between coeliac disease and enteropathy associated T cell lymphoma. Scand. J. Gastroenterol. Suppl. 2000, 32–37 (2000).
Abolhassani, H. et al. A review on guidelines for management and treatment of common variable immunodeficiency. Expert Rev. Clin. Immunol. 9, 561–574 (2013).
Rubio-Tapia, A., Talley, N. J., Gurudu, S. R., Wu, T. T. & Murray, J. A. Gluten-free diet and steroid treatment are effective therapy for most patients with collagenous sprue. Clin. Gastroenterol. Hepatol. 8, 344–349 (2010).
Gopal, P. & McKenna, B. J. The collagenous gastroenteritides: similarities and differences. Arch. Pathol. Lab. Med. 134, 1485–1489 (2010).
Gentile, N. M., Murray, J. A. & Pardi, D. S. Autoimmune enteropathy: a review and update of clinical management. Curr. Gastroenterol. Rep. 14, 380–385 (2012).
Masia, R., Peyton, S., Lauwers, G. Y. & Brown, I. Gastrointestinal biopsy findings of autoimmune enteropathy: a review of 25 cases. Am. J. Surg. Pathol. 38, 1319–1329 (2014).
Bhat, N., Anupama, N. K., Yelsangikar, A. & Vizhi, K. Olmesartan-related sprue-like enteropathy. Indian J. Gastroenterol. 33, 564–567 (2014).
Heerasing, N., Hair, C. & Wallace, S. Olmesartan-induced enteropathy. Intern. Med. J. 45, 117–118 (2015).
van Beurden, Y. H., Nijeboer, P., Janssen, J., Verbeek, W. H. & Mulder, C. J. Diarrhoea and malabsorption due to olmesartan use [Dutch]. Ned. Tijdschr. Geneeskd. 158, A7370 (2014).
Verbeek, W. H. et al. Incidence of enteropathy—associated T-cell lymphoma: a nation-wide study of a population-based registry in The Netherlands. Scand. J. Gastroenterol. 43, 1322–1328 (2008).
Van Weyenberg, S. J., Smits, F., Jacobs, M. A., Van Turenhout, S. T. & Mulder, C. J. Video capsule endoscopy in patients with nonresponsive celiac disease. J. Clin. Gastroenterol. 47, 393–399 (2012).
Hadithi, M. et al. The value of double-balloon enteroscopy in patients with refractory celiac disease. Am. J. Gastroenterol. 102, 987–996 (2007).
Mallant, M. et al. Abdominal computed tomography in refractory coeliac disease and enteropathy associated T-cell lymphoma. World J. Gastroenterol. 13, 1696–1700 (2007).
Van Weyenberg, S. J. et al. Double-balloon endoscopy as the primary method for small-bowel video capsule endoscope retrieval. Gastrointest. Endosc. 71, 535–541 (2010).
Van Weyenberg, S. J. et al. Comparison of MR enteroclysis with video capsule endoscopy in the investigation of small-intestinal disease. Abdom. Imaging 38, 42–51 (2013).
Daum, S. et al. Capsule endoscopy in refractory celiac disease. Endoscopy 39, 455–458 (2007).
Hadithi, M. et al. 18F-FDG PET versus CT for the detection of enteropathy-associated T-cell lymphoma in refractory celiac disease. J. Nucl. Med. 47, 1622–16227 (2006).
Nijeboer P, van Wanrooij, R. L., Tack, G. J., Mulder, C. J. & Bouma, G. Update on the diagnosis and management of refractory coeliac disease. Gastroenterol. Res. Pract. 2013, 518483 (2013).
van Wanrooij, R. L. et al. Accurate classification of RCD requires flow cytometry. Gut 59, 1732 (2010).
van Wanrooij, R. L. et al. Optimal strategies to identify aberrant intra-epithelial lymphocytes in refractory coeliac disease. J. Clin. Immunol. 34, 828–835 (2014).
Brar, P., Lee, S., Lewis, S., Egbuna, I., Bhagat, G. & Green, P. H. Budesonide in the treatment of refractory celiac disease. Am. J. Gastroenterol. 102, 2265–2269 (2007).
Jamma, S. et al. Small intestinal release mesalamine for the treatment of refractory celiac disease type I. J. Clin. Gastroenterol. 45, 30–33 (2011).
Goerres, M. S. et al. Azathioprine and prednisone combination therapy in refractory coeliac disease. Aliment Pharmacol. Ther. 18, 487–494 (2003).
Gillett, H. R. et al. Successful infliximab treatment for steroid-refractory celiac disease: a case report. Gastroenterology 122, 800–805 (2002).
Costantino, G. et al. Treatment of life-threatening type I refractory coeliac disease with long-term infliximab. Dig. Liver Dis. 40, 74–77 (2008).
Tack, G. J., van Asseldonk, D. P., van Wanrooij, R. L., van Bodegraven, A. A. & Mulder, C. J. Tioguanine in the treatment of refractory coeliac disease—a single centre experience. Aliment. Pharmacol. Ther. 36, 274–281 (2012).
Malamut, G. et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology 136, 81–90 (2009).
Al-Toma, A. et al. Cladribine therapy in refractory celiac disease with aberrant T cells. Clin. Gastroenterol. Hepatol. 4, 1322–1327 (2006).
Robak, T., Wierzbowska, A. & Robak, E. Recent clinical trials of cladribine in hematological malignancies and autoimmune disorders. Rev. Recent Clin. Trials 1, 15–34 (2006).
Al-Toma, A. et al. Cladribine therapy in refractory celiac disease with aberrant T cells. Clin. Gastroenterol. Hepatol. 4, 1322–1327 (2006).
Tack, G. J. et al. Evaluation of Cladribine treatment in refractory celiac disease type II. World J. Gastroenterol. 17, 506–513 (2011).
Al-Toma, A., Nijeboer, P., Bouma, G., Visser, O. & Mulder, C. J. Hematopoietic stem cell transplantation for non-malignant gastrointestinal diseases. World J. Gastroenterol. 20, 17368–17375 (2014).
Al-Toma, A. et al. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood 109, 2243–2249 (2007).
Tack, G. J. et al. Auto-SCT in refractory celiac disease type II patients unresponsive to cladribine therapy. Bone Marrow Transplant 46, 840–846 (2011).
Wahab, P. J., Crusius, J. B., Meijer, J. W., Uil, J. J. & Mulder, C. J. Cyclosporin in the treatment of adults with refractory coeliac disease—an open pilot study. Aliment. Pharmacol. Ther. 14, 767–774 (2000).
Al-Toma, A., Verbeek, W. H., Hadithi M, von Blomberg, B. M. & Mulder, C. J. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut 56, 1373–1378 (2007).
Gale, J., Simmonds, P. D., Mead, G. M., Sweetenham, J. W. & Wright, D. H. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J. Clin. Oncol. 18, 795–803 (2000).
van de Water, J. M. et al. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract. Res. Clin. Gastroenterol. 24, 43–56 (2010).
Nijeboer, P. et al. Therapy in RCDII: Rationale for Combination Strategies? Dig. Dis. 33, 227–230 (2015).
Malamut, G. et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J. Clin. Invest. 120, 2131–2143 (2010).
van der, H. D. et al. Tofacitinib (CP-690550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 65, 559–570 (2013).
van Vollenhoven, R. F. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 367, 508–519 (2012).
Author information
Authors and Affiliations
Contributions
All authors made equal contributions to writing the article. G.B. and C.J.J.M. made substantial contributions to discussion of content and reviewed/edited the manuscript before submission. P.N., R.L.v.W., G.B. and C.J.J.M. researched data for the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
van Gils, T., Nijeboer, P., van Wanrooij, R. et al. Mechanisms and management of refractory coeliac disease. Nat Rev Gastroenterol Hepatol 12, 572–579 (2015). https://doi.org/10.1038/nrgastro.2015.155
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.155
This article is cited by
-
Enteric-Release Budesonide May Be Useful in the Management of Non-Responsive Celiac Disease
Digestive Diseases and Sciences (2021)
-
Immunosuppression-induced clonal T-cell lymphoproliferative disease causing severe diarrhoea mimicking coeliac disease following renal transplantation: a case report
BMC Nephrology (2020)
-
How to manage celiac disease and gluten-free diet during the COVID-19 era: proposals from a tertiary referral center in a high-incidence scenario
BMC Gastroenterology (2020)
-
Systematic Literature Review of the Economic Burden of Celiac Disease
PharmacoEconomics (2019)
-
Transglutaminase 2 and Transglutaminase 2 Autoantibodies in Celiac Disease: a Review
Clinical Reviews in Allergy & Immunology (2019)