Key Points
-
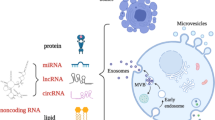
Microvesicles (MVs) are 0.1–1.0 μm vesicles containing lipids, proteins, RNAs and microRNAs; they are formed by budding from the cellular plasma membrane
-
Circulating levels of several subpopulations of MVs are increased in patients with liver diseases, probably due to enhanced MV production and decreased MV clearance, related to the individual liver disorder
-
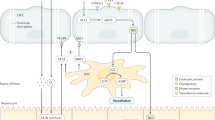
MVs are now implicated at many stages of liver disease progression, including liver fibrogenesis, portal hypertension and activation of coagulation
-
Several results suggest that MVs have a role in hepatocellular carcinoma by conveying information between tumour cells and between tumour and neighbouring cells
-
High levels of circulating procoagulant MVs have been found in patients with acute liver failure and might contribute to normal or hypercoagulable global haemostasis in this setting
-
MVs have promise as diagnostic and prognostic biomarkers in patients with liver diseases
Abstract
Microvesicles (MVs) are extracellular vesicles released by virtually all cells, under both physiological and pathological conditions. They contain lipids, proteins, RNAs and microRNAs and act as vectors of information that regulate the function of target cells. This Review provides an overview of the studies assessing circulating MV levels in patients with liver diseases, together with an insight into the mechanisms that could account for these changes. We also present a detailed analysis of the implication of MVs in key processes of liver diseases. MVs have a dual role in fibrosis as certain types of MVs promote fibrolysis by increasing expression of matrix metalloproteinases, whereas others promote fibrosis by stimulating processes such as angiogenesis. MVs probably enhance portal hypertension by contributing to intrahepatic vasoconstriction, splanchnic vasodilation and angiogenesis. As MVs can modulate vascular permeability, vascular tone and angiogenesis, they might contribute to several complications of cirrhosis including hepatic encephalopathy, hepatopulmonary syndrome and hepatorenal syndrome. Several results also suggest that MVs have a role in hepatocellular carcinoma. Although MVs represent promising biomarkers in patients with liver disease, methods of isolation and subsequent analysis must be standardized.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Witwer, K. W. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 20360 (2013).
van der Pol, E., Boing, A. N., Harrison, P., Sturk, A. & Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 64, 676–705 (2012).
Connor, D. E., Exner, T., Ma, D. D. & Joseph, J. E. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb. Haemost. 103, 1044–1052 (2010).
Mause, S. F. & Weber, C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 107, 1047–1057 (2010).
Rautou, P. E. & Mackman, N. Deletion of microvesicles from the circulation. Circulation 125, 1601–1604 (2012).
Bernimoulin, M. et al. Differential stimulation of monocytic cells results in distinct populations of microparticles. J. Thromb. Haemost. 7, 1019–1028 (2009).
Leroyer, A. S. et al. CD40 ligand microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J. Am. Coll. Cardiol. 52, 1302–1311 (2008).
Berckmans, R. J., Sturk, A., van Tienen, L. M., Schaap, M. C. & Nieuwland, R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood 117, 3172–3180 (2011).
Rood, I. M. et al. Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome. Kidney Int. 78, 810–816 (2010).
Witek, R. P. et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology 136, 320–330 (2009).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010).
Chahed, S. et al. Increased vitreous shedding of microparticles in proliferative diabetic retinopathy stimulates endothelial proliferation. Diabetes 59, 694–701 (2010).
Franz, C. et al. Procoagulant tissue factor-exposing vesicles in human seminal fluid. J. Reprod. Immunol. 98, 45–51 (2013).
Leroyer, A. S. et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation 119, 2808–2817 (2009).
Baron, M. et al. PPARα activation differently affects microparticle content in atherosclerotic lesions and liver of a mouse model of atherosclerosis and NASH. Atherosclerosis 218, 69–76 (2011).
Amabile, N., Rautou, P. E., Tedgui, A. & Boulanger, C. M. Microparticles: key protagonists in cardiovascular disorders. Semin. Thromb. Hemost. 36, 907–916 (2010).
Rautou, P. E. & Mackman, N. Microvesicles as risk markers for venous thrombosis. Expert Rev. Hematol. 6, 91–101 (2013).
Zahra, S., Anderson, J. A., Stirling, D. & Ludlam, C. A. Microparticles, malignancy and thrombosis. Br. J. Haematol. 152, 688–700 (2011).
Fusegawa, H. et al. Platelet activation in patients with chronic hepatitis C. Tokai J. Exp. Clin. Med. 27, 101–106 (2002).
Brodsky, S. V. et al. Dynamics of circulating microparticles in liver transplant patients. J. Gastrointestin. Liver Dis. 17, 261–268 (2008).
Sayed, D., Amin, N. F. & Galal, G. M. Monocyte-platelet aggregates and platelet micro-particles in patients with post-hepatitic liver cirrhosis. Thromb. Res. 125, e228–e233 (2010).
Kornek, M., Popov, Y., Libermann, T. A., Afdhal, N. H. & Schuppan, D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology 53, 230–242 (2011).
Ogasawara, F. et al. Platelet activation in patients with alcoholic liver disease. Tokai J. Exp. Clin. Med. 30, 41–48 (2005).
Rautou, P. E. et al. Abnormal plasma microparticles impair vasoconstrictor responses in patients with cirrhosis. Gastroenterology 143, 166–176 (2012).
Kornek, M. et al. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology 143, 448–458 (2012).
Esch, J. S. et al. Platelet activation and increased tissue factor expression on monocytes in reperfusion injury following orthotopic liver transplantation. Platelets 21, 348–359 (2010).
Agarwal, B. et al. Evaluation of coagulation abnormalities in acute liver failure. J. Hepatol. 57, 780–786 (2012).
Agarwal, B. et al. Hemostasis in patients with acute kidney injury secondary to acute liver failure. Kidney Int. 84, 158–163 (2013).
Stravitz, R. T. et al. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/acute liver failure. Hepatology 58, 304–313 (2013).
Schmelzle, M. et al. Increased plasma levels of microparticles expressing CD39 and CD133 in acute liver injury. Transplantation 95, 63–69 (2013).
Chen, Y., Davis-Gorman, G., Watson, R. R. & McDonagh, P. F. Platelet CD62p expression and microparticle in murine acquired immune deficiency syndrome and chronic ethanol consumption. Alcohol 38, 25–30 (2003).
Meckes, D. G. Jr & Raab-Traub, N. Microvesicles and viral infection. J. Virol. 85, 12844–12854 (2011).
Diamant, M. et al. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 106, 2442–2447 (2002).
Esposito, K. et al. Endothelial microparticles correlate with endothelial dysfunction in obese women. J. Clin. Endocrinol. Metab. 91, 3676–3679 (2006).
Ferreira, A. C. et al. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation 110, 3599–3603 (2004).
Navasiolava, N. M. et al. Enforced physical inactivity increases endothelial microparticle levels in healthy volunteers. Am. J. Physiol. Heart Circ. Physiol. 299, H248–H256 (2010).
Guicciardi, M. E. & Gores, G. J. Apoptosis as a mechanism for liver disease progression. Semin. Liver Dis. 30, 402–410 (2010).
Violi, F. et al. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J. Hepatol. 55, 1415–1427 (2011).
Miyoshi, H. et al. Calpain activation in plasma membrane bleb formation during tert-butyl hydroperoxide-induced rat hepatocyte injury. Gastroenterology 110, 1897–1904 (1996).
Cazzaniga, M. et al. Increased flow-mediated vasodilation in cirrhotic patients with ascites: relationship with renal resistive index. Liver Int. 28, 1396–1401 (2008).
Tazi, K. A. et al. Role of shear stress in aortic eNOS up-regulation in rats with biliary cirrhosis. Gastroenterology 122, 1869–1877 (2002).
Rasaratnam, B., Kaye, D., Jennings, G., Dudley, F. & Chin-Dusting, J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann. Intern. Med. 139, 186–193 (2003).
Nomura, S., Nakamura, T., Cone, J., Tandon, N. N. & Kambayashi, J. Cytometric analysis of high shear-induced platelet microparticles and effect of cytokines on microparticle generation. Cytometry 40, 173–181 (2000).
Abid Hussein, M. N. et al. Cell-derived microparticles contain caspase 3 in vitro and in vivo. J. Thromb. Haemost. 3, 888–896 (2005).
Essayagh, S. et al. Microparticles from apoptotic vascular smooth muscle cells induce endothelial dysfunction, a phenomenon prevented by β3-integrin antagonists. Thromb. Haemost. 94, 853–858 (2005).
Szotowski, B. et al. Antioxidative treatment inhibits the release of thrombogenic tissue factor from irradiation- and cytokine-induced endothelial cells. Cardiovasc. Res. 73, 806–812 (2007).
Liu, M. L., Reilly, M. P., Casasanto, P., McKenzie, S. E. & Williams, K. J. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler. Thromb. Vasc. Biol. 27, 430–435 (2007).
Li, D. et al. TLR4 signaling induces the release of microparticles by tumor cells that regulate inflammatory cytokine IL-6 of macrophages via microRNA let-7b. Oncoimmunology 1, 687–693 (2012).
Povero, D. et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require vanin-1 for uptake by endothelial cells. Sci. Signal. 6, ra88 (2013).
Al Faraj, A. et al. Endothelial cell-derived microparticles loaded with iron oxide nanoparticles: feasibility of MR imaging monitoring in mice. Radiology 263, 169–178 (2012).
Willekens, F. L. et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood 105, 2141–2145 (2005).
Gomez, F., Ruiz, P. & Schreiber, A. D. Impaired function of macrophage Fc γ receptors and bacterial infection in alcoholic cirrhosis. N. Engl. J. Med. 331, 1122–1128 (1994).
Chen, L., Pan, D. D., Zhou, J. & Jiang, Y. Z. Protective effect of selenium-enriched Lactobacillus on CCl4-induced liver injury in mice and its possible mechanisms. World J. Gastroenterol. 11, 5795–5800 (2005).
Rimola, A. et al. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology 4, 53–58 (1984).
Tanimoto, A. et al. Superparamagnetic iron oxide-mediated hepatic signal intensity change in patients with and without cirrhosis: pulse sequence effects and Kupffer cell function. Radiology 222, 661–666 (2002).
Dasgupta, S. K., Le, A., Chavakis, T., Rumbaut, R. E. & Thiagarajan, P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation 125, 1664–1672 (2012).
Distler, J. H. et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl Acad. Sci. USA 102, 2892–2897 (2005).
Taraboletti, G. et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 160, 673–680 (2002).
Gasser, O. et al. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell. Res. 285, 243–257 (2003).
Szabo, G. & Bala, S. MicroRNAs in liver disease. Nat. Rev. Gastroenterol. Hepatol. 10, 542–552 (2013).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Diehl, P. et al. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc. Res. 93, 633–644 (2012).
Cantaluppi, V. et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 82, 412–427 (2012).
Thabut, D. & Shah, V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J. Hepatol. 53, 976–980 (2010).
Mause, S. F. et al. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation 122, 495–506 (2010).
Benameur, T., Soleti, R., Porro, C., Andriantsitohaina, R. & Martinez, M. C. Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS ONE 5, e12688 (2010).
Deregibus, M. C. et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110, 2440–2448 (2007).
Friedman, S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172 (2008).
Thabut, D. et al. High-density lipoprotein administration attenuates liver proinflammatory response, restores liver endothelial nitric oxide synthase activity, and lowers portal pressure in cirrhotic rats. Hepatology 46, 1893–1906 (2007).
Aram, G., Potter, J. J., Liu, X., Torbenson, M. S. & Mezey, E. Lack of inducible nitric oxide synthase leads to increased hepatic apoptosis and decreased fibrosis in mice after chronic carbon tetrachloride administration. Hepatology 47, 2051–2058 (2008).
Nieuwland, R. et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood 95, 930–935 (2000).
Owens, A. P. 3rd & Mackman, N. Microparticles in hemostasis and thrombosis. Circ. Res. 108, 1284–1297 (2011).
Valla, D. C. Thrombosis and anticoagulation in liver disease. Hepatology 47, 1384–1393 (2008).
Teoh, N. C. et al. Diannexin, a novel annexin V homodimer, provides prolonged protection against hepatic ischemia–reperfusion injury in mice. Gastroenterology 133, 632–646 (2007).
Garcia-Pagan, J. C., Gracia-Sancho, J. & Bosch, J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J. Hepatol. 57, 458–461 (2012).
Lemoinne, S. et al. Portal myofibroblasts promote liver angiogenesis through the release of microparticles and interaction with cholangiocytes. Hepatology 56, 304A–305A (2012).
Schwartz, D. et al. Inhibition of constitutive nitric oxide synthase (NOS) by nitric oxide generated by inducible NOS after lipopolysaccharide administration provokes renal dysfunction in rats. J. Clin. Invest. 100, 439–448 (1997).
Mostefai, H. A. et al. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am. J. Respir. Crit. Care Med. 178, 1148–1155 (2008).
Melgar-Lesmes, P. et al. Vascular endothelial growth factor and angiopoietin-2 play a major role in the pathogenesis of vascular leakage in cirrhotic rats. Gut 58, 285–292 (2009).
Densmore, J. C. et al. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock 26, 464–471 (2006).
Perez-Casal, M. et al. Microparticle-associated endothelial protein C receptor and the induction of cytoprotective and anti-inflammatory effects. Haematologica 94, 387–394 (2009).
Tripodi, A. & Mannucci, P. M. The coagulopathy of chronic liver disease. N. Engl. J. Med. 365, 147–156 (2011).
Press, J. Z. et al. Microparticles from ovarian carcinomas are shed into ascites and promote cell migration. Int. J. Gynecol. Cancer 22, 546–552 (2012).
Ginestra, A., Miceli, D., Dolo, V., Romano, F. M. & Vittorelli, M. L. Membrane vesicles in ovarian cancer fluids: a new potential marker. Anticancer Res. 19, 3439–3445 (1999).
Choi, D. S. et al. Proteomic analysis of microvesicles derived from human colorectal cancer ascites. Proteomics 11, 2745–2751 (2011).
Cejudo-Martin, P. et al. Hypoxia is an inducer of vasodilator agents in peritoneal macrophages of cirrhotic patients. Hepatology 36, 1172–1179 (2002).
Jimenez, W. et al. Nitric oxide production and inducible nitric oxide synthase expression in peritoneal macrophages of cirrhotic patients. Hepatology 30, 670–676 (1999).
Morales-Ruiz, M. et al. Ascites from cirrhotic patients induces angiogenesis through the phosphoinositide 3-kinase/Akt signaling pathway. J. Hepatol. 43, 85–91 (2005).
Wright, G. & Jalan, R. Ammonia and inflammation in the pathogenesis of hepatic encephalopathy: Pandora's box? Hepatology 46, 291–294 (2007).
Wright, G. et al. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology 45, 1517–1526 (2007).
Jayakumar, A. R., Tong, X. Y., Ospel, J. & Norenberg, M. D. Role of cerebral endothelial cells in the astrocyte swelling and brain edema associated with acute hepatic encephalopathy. Neuroscience 218, 305–316 (2012).
Rodriguez-Roisin, R. & Krowka, M. J. Hepatopulmonary syndrome—a liver-induced lung vascular disorder. N. Engl. J. Med. 358, 2378–2387 (2008).
Amabile, N. et al. Cellular microparticles in the pathogenesis of pulmonary hypertension. Eur. Respir. J. 42, 272–279 (2013).
Camus, S. M. et al. Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood 120, 5050–5058 (2012).
Tripodi, A. et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology 137, 2105–2111 (2009).
Tripodi, A., Anstee, Q. M., Sogaard, K. K., Primignani, M. & Valla, D. C. Hypercoagulability in cirrhosis: causes and consequences. J. Thromb. Haemost. 9, 1713–1723 (2011).
Tripodi, A., Primignani, M., Lemma, L., Chantarangkul, V. & Mannucci, P. M. Evidence that low protein C contributes to the procoagulant imbalance in cirrhosis. J. Hepatol. 59, 265–270 (2013).
Wiest, R. & Garcia-Tsao, G. Bacterial translocation (BT) in cirrhosis. Hepatology 41, 422–433 (2005).
Rautou, P.-E., Vion, A.-C., Luyendyk, J. P. & Mackman, N. Microparticle tissue factor activity is increased in patients with cirrhosis. Hepatology (in press).
Stravitz, R. T. et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J. Hepatol. 56, 129–136 (2012).
Ganey, P. E. et al. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology 46, 1177–1186 (2007).
Zafrani, L. et al. Calpastatin controls polymicrobial sepsis by limiting procoagulant microparticle release. Am. J. Respir. Crit. Care Med. 185, 744–755 (2012).
Thaler, J. et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J. Thromb. Haemost. 10, 1363–1370 (2012).
Rak, J. Microparticles in cancer. Semin. Thromb. Hemost. 36, 888–906 (2010).
Al-Nedawi, K., Meehan, B., Kerbel, R. S., Allison, A. C. & Rak, J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl Acad. Sci. USA 106, 3794–3799 (2009).
Al-Nedawi, K. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624 (2008).
Jaiswal, R. et al. Microparticle-associated nucleic acids mediate trait dominance in cancer. FASEB J. 26, 420–429 (2012).
Shedden, K., Xie, X. T., Chandaroy, P., Chang, Y. T. & Rosania, G. R. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 63, 4331–4337 (2003).
Sidhu, S. S., Mengistab, A. T., Tauscher, A. N., LaVail, J. & Basbaum, C. The microvesicle as a vehicle for EMMPRIN in tumor–stromal interactions. Oncogene 23, 956–963 (2004).
Ma, J. et al. Innate immune cell-derived microparticles facilitate hepatocarcinoma metastasis by transferring integrin α(M)β(2) to tumor cells. J. Immunol. 191, 3453–3461 (2013).
Fonsato, V. et al. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells 30, 1985–1998 (2012).
Villa, E. et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 143, 1253–1260 (2012).
Hu, J. et al. Plasma microRNA, a potential biomarker for acute rejection after liver transplantation. Transplantation 95, 991–999 (2013).
Lacroix, R. et al. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J. Thromb. Haemost. 11, 1190–1193 (2013).
European Association of National Metrology Institutes (EURA-MET). Metrological characterisation of micro-vesicles from body fluids as non-invasive diagnostic biomarkers [online], (2014).
European Cooperation in Science and Technology (COST). European Network on Microvesicles and Exosomes in Health and Disease (ME-HAD) [online], (2012).
Herrera, M. B. et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J. Cell. Mol. Med. 14, 1605–1618 (2010).
Acknowledgements
This authors of this Review receive funding for laboratory research from the Société Nationale Française de Gastroentérologie (SNFGE), the Association Française pour l'Étude du Foie (AFEF) and the Agence Nationale pour la Recherche (ANR-12-EMMA-0012-03).
Author information
Authors and Affiliations
Contributions
S. Lemoinne and P.-E. Rautou contributed to all aspects of the preparation of this manuscript. D. Thabut, C. Housset, R. Moreau, D. Valla and C. M. Boulanger contributed to discussion of content and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lemoinne, S., Thabut, D., Housset, C. et al. The emerging roles of microvesicles in liver diseases. Nat Rev Gastroenterol Hepatol 11, 350–361 (2014). https://doi.org/10.1038/nrgastro.2014.7
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.7
This article is cited by
-
CINC-2 and miR-199a-5p in EVs secreted by transplanted Thy1+ cells activate hepatocytic progenitor cell growth in rat liver regeneration
Stem Cell Research & Therapy (2023)
-
Diagnosis of portal vein thrombosis in cirrhotic patients with and without hepatocellular carcinoma
Egyptian Liver Journal (2022)
-
Anticoagulation in patients with advanced liver disease: an open issue
Internal and Emergency Medicine (2021)
-
Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure
Hepatology International (2021)
-
Extraction and identification of synovial tissue-derived exosomes by different separation techniques
Journal of Orthopaedic Surgery and Research (2020)